Question
Question: Which of the following groups is sharp ortho and para directive? A) \( - {{\text{C}}_6}{{\text{H}}...
Which of the following groups is sharp ortho and para directive?
A) −C6H5
B) −OH
C) −CH3
D) −Cl
Solution
Here, all the groups are ortho and para directing as they are all electron donating groups .The −OH is the sharp ortho and para directing group as the lone pair present on oxygen group increase electron density at ortho and para positions.
Step-by-Step Explanation:
When mono-substituted benzene reacts with an electrophile, the electrophile can get attached to ortho, para or meta position on benzene. The electrophile if gets attached to the ortho or para position, it is called the ortho and para directing group. If it gets attached to the meta position it is called a meta directing group. The ortho and para directing groups are electron donating groups. Here, given all options are ortho and para directing. But −OH group is sharp ortho and para directing as the lone pair electron present in the Oxygen atom of the group increases electron density at ortho and para position so that the electrophile can easily get attached at these positions. The resonance structure of phenol is as follows-
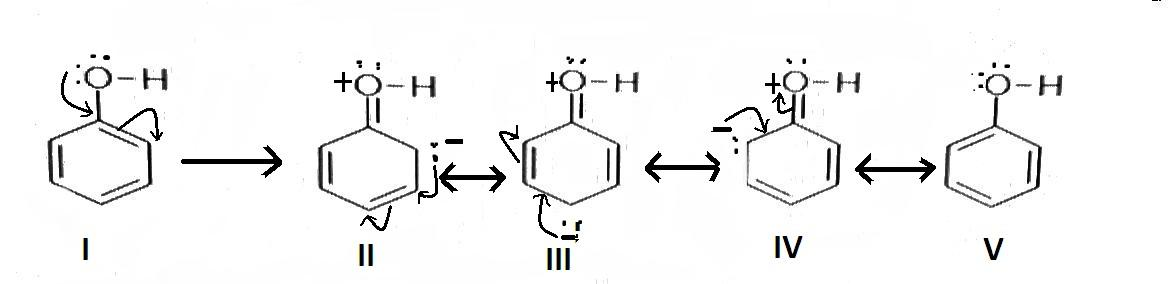
Hence correct answer is ‘B’.
Note: While ortho and para directing groups are electron donating groups, the meta directing groups are electron withdrawing groups. Halides are the exception of this rule because even when they are electron withdrawing groups they are still ortho and para directing.
