Question
Question: Which of the following forms metaldehyde as a major product? A.\[4C{H_3}CHO\xrightarrow{{Conc{H_2}...
Which of the following forms metaldehyde as a major product?
A.4CH3CHOConcH2SO4,−100C
B.CH3COCH3ConcH2SO4,roomtemp
C.3CH3CHOConcH2SO4,roomtemp
D.C6H5CHOConcH2SO4,
Solution
Metaldehyde is an organic compound with the molecular formula of (CH3CHO)4 . It can be formed by the reaction of four molecules of acetaldehyde with concentrated sulphuric acid at a temperature below zero degree Celsius.
Complete answer:
Chemical compounds are classified into functional groups based on the groups present in them. Carbonyl compounds are classified into aldehydes and ketones. In aldehyde, the carbonyl group is present between the one hydrogen atom and one methyl group. Whereas ketone is a functional group consisting of a functional group that is present in between the two alkyl parts or carbon atom that is a part of the alkyl or phenyl group.
When four molecules of acetaldehyde were treated with concentrated sulphuric acid at −100C , it results in the formation of an organic molecule which is known as a cyclic tetramer of aldehyde.
The chemical reaction involving the formation of a cyclic tetramer of aldehyde is as follows:
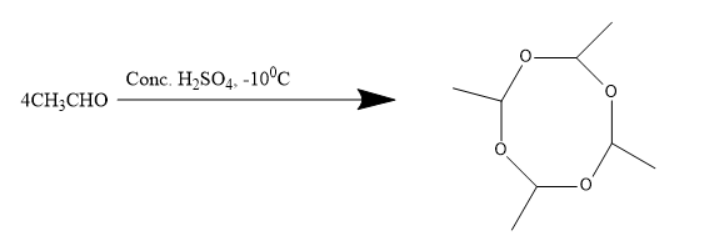
The compound formed above is known as metaldehyde. It can be formed by the reaction of four molecules of acetaldehyde. Thus, metaldehyde is formed by 4CH3CHO
Thus, option A is the correct answer.
Note:
When the four molecules of acetaldehyde were reacted with Concentrated Sulphuric acid resulted in metaldehyde. The temperature needed is below zero degrees Celsius. Whereas three molecules of acetaldehyde were reacted with Concentrated sulphuric acid results in the tricyclic organic compound. The room temperature is needed for this reaction.
