Question
Question: Which of the following fluoro-compounds is most likely to behave as a Lewis base? A. \[{\text{C}}{...
Which of the following fluoro-compounds is most likely to behave as a Lewis base?
A. CF4
B. SiF4
C. BF3
D. PF3
Solution
A Lewis base is electron-rich species. It tends to donate its electron pair. Draw the Lewis dot structures of all given molecules and determine which molecule has the lone pair of electrons to donate.
Complete answer:
A Lewis base is the species that can donate a pair of electrons.
Now, to determine which of fluoro -compounds is most likely to behave as a Lewis base we will draw the Lewis dot structures of all given fluoro –compounds.
A. CF4
To draw the Lewis dot structure we have to determine the total number of valence electrons.
Carbon is a Group IVA element so it has 4 valence electrons. Fluorine is a group VIIA element so it has 7 valence electrons.
So, total valence electrons = 4 + 7(4) = 32
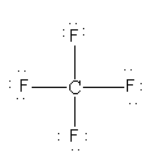
As there is no lone pair on the central carbon atom so CF4 cannot behave as a Lewis base.
Thus, option (A) CF4 is incorrect.
B. SiF4
Silicon is a Group IVA element so it has 4 valence electrons. Fluorine is a group VIIA element so it has 7 valence electrons.
So, total valence electrons = 4 + 7(4) = 32
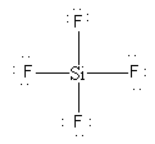
As there is no lone pair on the central silicon atom so SiF4cannot behave as a Lewis base.
Thus, option (B) SiF4 is incorrect.
C. BF3
Boron is a group IIIA element so it has 3 valence electrons. Fluorine is a group VIIA element so it has 7 valence electrons.
So, total valence electrons = 3 + 7(3) = 24
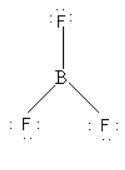
As there is no lone pair on the central boron atom so BF3cannot behave as a Lewis base.
Thus, option (C) BF3 is incorrect.
D. PF3
Phosphorus is a group VA element so it has 5 valence electrons. Fluorine is a group VIIA element so it has 7 valence electrons.
So, total valence electrons = 5 + 7(3) = 26
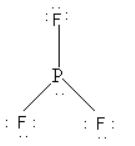
As there is a lone pair on the central phosphorus atom. So PF3 tends to donate its lone pair of electrons and behave as Lewis base.
**Thus, option (D) PF3 is correct.
Note:**
Lewis bases are also known as nucleophiles as they are electron-rich species. The number of valence electrons of an element is nothing but its group number. While drawing Lewis dot structures always first distribute the electrons as lone pairs on the surrounding atom to complete their octet. After completing the octet of the surrounding atom we can distribute the remaining electrons as a lone pair on the central atom to complete the octet of the central atom.
