Question
Question: Which of the following ethers is the most unreactive to cleavage with conc. HBr? A.
B.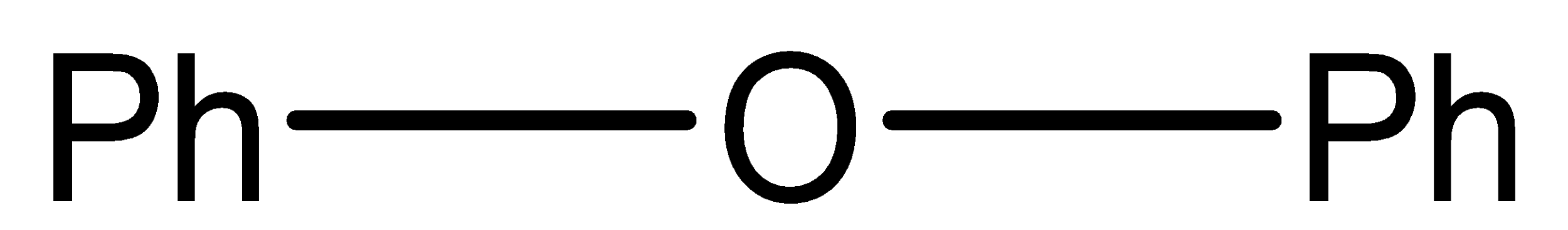
C.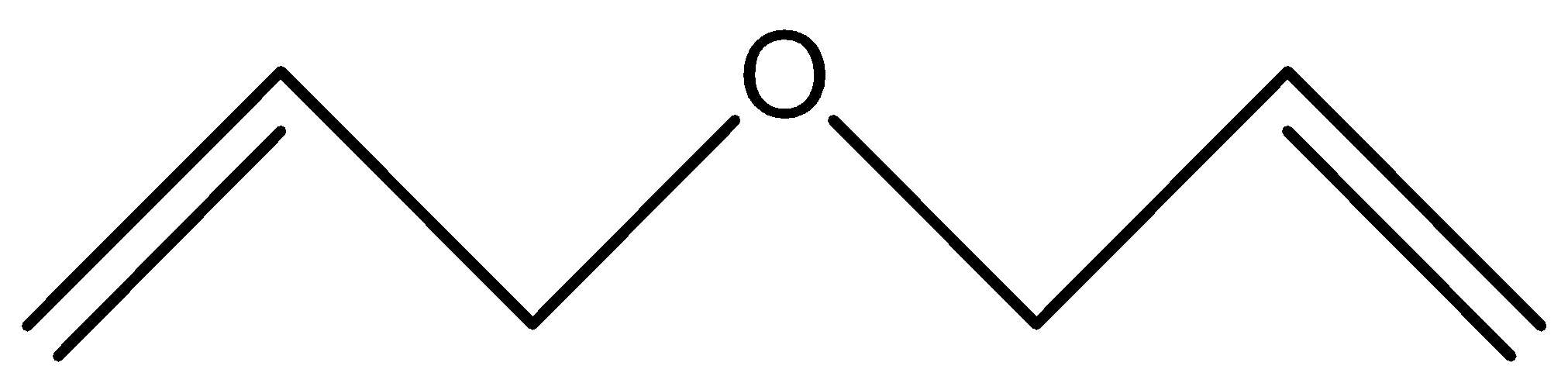
D.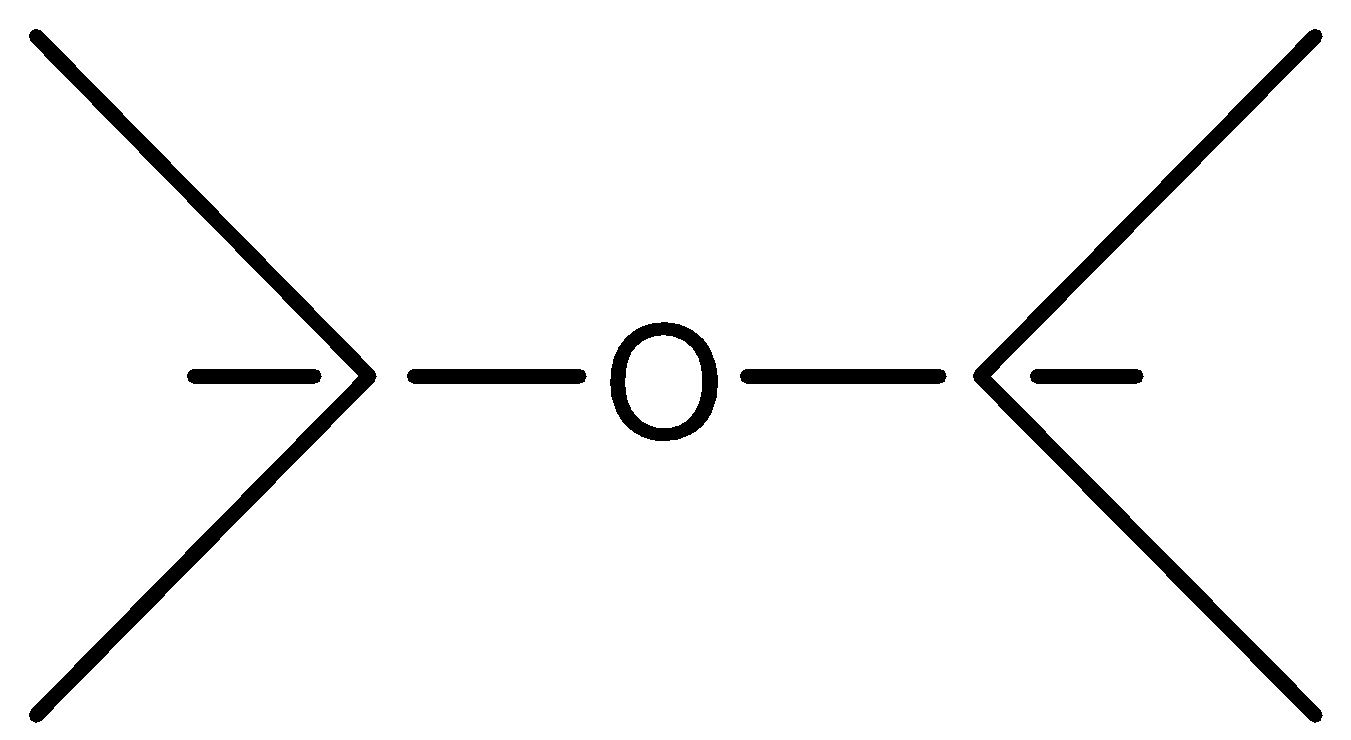
Solution
Diphenyl ether cannot be cleaved by concentrated HBr, it is because in diphenyl ether Oxygen (O) is attached to both phenyl ring and thus the nucleophilic attack is not feasible and also positive charge on benzene unstable.
Complete step by step answer:
Diphenyl ether is the most stable one, and to break bond with phenyl required larger energy.
Alkyl ethers are cleaved by the strong acids that are HI (Hydrogen iodide) or HBr (Hydrogen Bromide) in nucleophilic substitution reactions similar to alcohols. Protonation of the ethereal oxygen creates a good leaving group, a neutral alcohol molecule. The halide ion, bromide or iodide are both good nucleophiles. Considering the structure of the alkyl groups, the reaction can be SN1 or SN2 like. Ethers are highly stable and cannot be claimed or dissociated by HBr or HI, So, it needs concentrated HBr or with dilute H2SO4 phenyl attached with ether has an invisible double bond with O and it forms a very unstable product if O is removed and so as we know every compounds need to be stable so diphenyl ether least reactive to HBr because of its Phenyl.
So, the correct answer is option (B).
Note: In an acid/base reaction, protonation of the alcoholic oxygen to make a better leaving group. This step is very fast and reversible. The lone pairs on the oxygen make it a Lewis base. The bromide ion functions as the nucleophile and attacks to displace the good leaving group, neutral alcohol molecule, by cleaving the C−O bond. This results in the formation of an alkyl bromide and an alcohol.
