Question
Question: Which of the following enhances the lathering property of soap? A. Sodium carbonate B. Sodium ro...
Which of the following enhances the lathering property of soap?
A. Sodium carbonate
B. Sodium rosinate
C. Sodium stearate
D. Trisodium phosphate
Solution
Soaps can be made with desirable properties for suitable uses by varying the reactants in the saponification reaction.
Complete step by step solution
The reaction of fatty acids with an alkali results in the formation of soaps. This reaction is called the saponification reaction. The structures of soaps are composed of long chains of molecules containing cationic and anionic parts.
We can define soaps that we use for cleansing purpose in simple words as salts of Na+orK+ with long chain fatty acids such as stearic acid or oleic acid. We know the process of formation of soap containing Na+ by heating the glyceryl ester of a fatty acid with NaOH(aq) as saponification. For example, using ester of stearic acid:
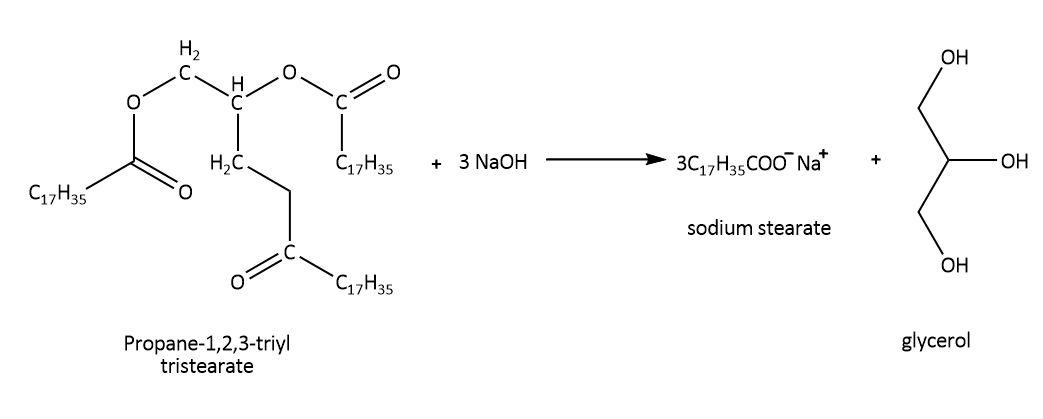
Now, this is a common soap but we can choose suitable raw material to enhance or modify the properties to get different types of soaps. For example, we can add some material that might induce some medicinal effects such as antibiotic soaps, we can also add color or perfumes to make them more attractive, in shaving soaps or laundry soaps we can add sodium rosinate which help them to lather well or we can also add glycerol to shaving soaps to help with skin drying. Sodium carbonate or borax can be used as fillers in laundry soaps
From the above discussion, we can conclude that it is sodium rosinate that can be used to enhance the lathering property of soaps.
**Hence, the correct option is B.
Note: **
We can have builders as well in soaps that help them for quick action and the examples include sodium carbonate and trisodium phosphate.
