Question
Question: Which of the following energy profile diagrams for a three-step reaction in which the first step is ...
Which of the following energy profile diagrams for a three-step reaction in which the first step is slowest and the last step is fastest? (Assume that reaction is exothermic)
A.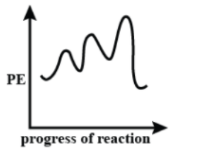
B.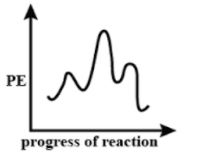
C.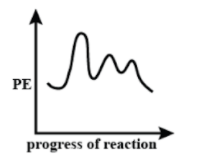
D.None of These
Solution
We need to know that an energy profile diagram is a plot of Energy v/s Reaction. It is mainly used to determine the Activation energy, transition state, enthalpy change, and type of the reaction (exothermic or endothermic).
Complete answer:
An energy profile diagram appears as shown below:
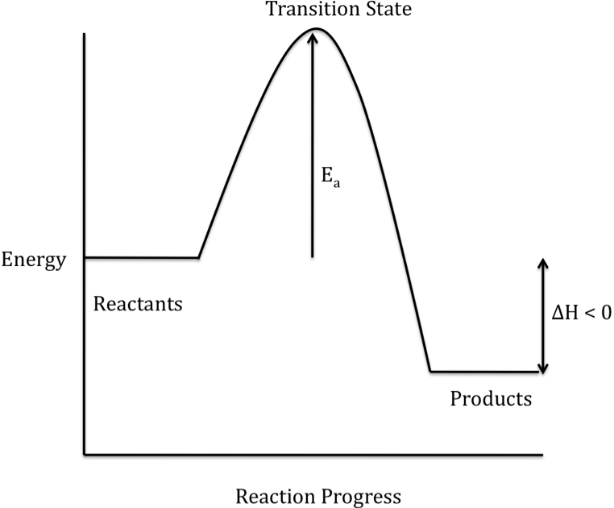
The activation energy is the highest point of the graph. For a reaction to proceed to completion, it has to overcome the energy barrier.
As the given reaction is a three-step process, the energy profile diagram will have 3 curves. The activation energy is the minimum energy required to overcome the energy barrier, i.e. to convert into reactants to products.
It is given that in the reaction, the first step is the slowest step, which means that it has the highest activation energy. Slowest step indicated that it requires a large amount of energy to overcome the barrier. Therefore, it will have the highest first curve in the energy profile diagram. The third step is the fastest step which means that it has the lowest activation energy, for the reactants to easily overcome it and convert into products. Hence the third curve will be the shortest of all.
These Conditions can be found in Graph Option (C). Hence Option (C) is the correct answer.
In Graph (A) the first curve is the shortest, which means that the first step is the fastest, which doesn’t satisfy the conditions in the question. Hence option (A) is incorrect.
In Graph (B) the second curve is the highest, which means that the second step is the slowest and the first curve is the shortest, which means that it is the fastest. Hence Option (B) is incorrect.
Hence Option (C) is the correct answer.
Note:
From the energy profile diagram, it can be determined whether the reaction is exothermic or endothermic. If the energy of products is lesser than that of reactants, which means that heat has been released, it is an exothermic Reaction.
