Question
Question: Which of the following electron configurations has maximum energy ? A.
B.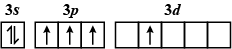
C.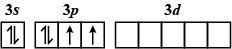
D.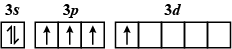
Solution
Energy of orbitals and shell: 1s is the least energy orbital and it is close to the nucleus. 1s is first then second shell, which consists of 2s and 2p. The second shell has high energy and is away from the nucleus than the first shell.
Complete step by step solution:
The order of increasing energy of the sub-atomic orbitals is s<p<d<f.
The energy in an excited state is more than that in the ground state.
So, the correct answer is A.
Additional information:
Each orbital in an atom is considered by a unique set of values of the three quantum numbers n,l and m, which resemble the electron's energy, angular momentum, and angular momentum vector components . Each orbital can be engaged by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum numbers having l=0,1,2,3 respectively. The names together along the value of n are used to describe the electron configurations of atoms. These are derived from the description by early spectroscopists of certain series of alkali metal .Orbitals for l>3 continue alphabetically.
Note: Atomic orbitals are known as the basic building blocks of the atomic orbital model which is also known as the electron cloud or wave mechanics model. It is a modern framework for visualizing the smallest behavior of electrons. In this model the electron cloud of a multi-electron atom is seen as being built up in an electron configuration which is a product of simpler hydrogen-like atomic orbitals.
