Question
Question: Which of the following doesn’t give yellow precipitate with \(AgN{{O}_{3}}\)? (A) \({{\left( C{{H}...
Which of the following doesn’t give yellow precipitate with AgNO3?
(A) (CH3)3C−CH2−I
(B) CH2=CH−CH2−I
(C) 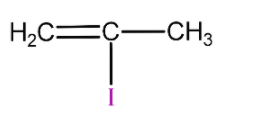
(D) 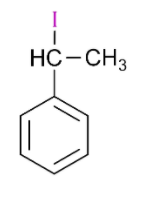
Solution
Think about the reaction which takes place when iodine derivatives react with silver nitrate. Also, take a look at the compounds given in the options and check which one is an exception to this reaction.
Complete step by step solution:
- When iodine derivatives react with silver nitrate, AgNO3, alkyl nitrate is formed which precipitates out as yellow ppt. The reaction is shown as follows:
R−I+AgNO3→R−ONO2+AgI
- This reaction is readily given by iodine derivatives of alkanes, alkenes, alkynes, allyl and benzyl groups.
- Vinyl iodide and phenyl iodide do not give this reaction due to the presence of strong C-I bond. They do not form any precipitate. Vinyl iodide has conjugate effect and phenyl iodide has resonance effect which stabilizes the bond and makes it stronger.
- Now, let’s take a look at all options given the question.
- (CH3)3C−CH2−I is an alkane so it will react with silver nitrate to form yellow precipitate.
- CH2=CH−CH2−I is allyl iodide, so it will also react with silver nitrate to form yellow precipitate.
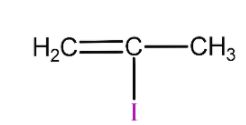
is 2-methyl vinyl iodide so it will not react with silver nitrate due to strong C-I bond and will not form yellow precipitate.
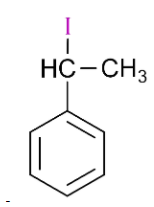
is 1-iodo-1-phenylethane, so it will also react with silver nitrate and form yellow precipitate.
- Therefore, option (C) is the correct answer.
Note: Remember all iodine derivatives except vinyl iodide and phenyl iodide, will react with silver nitrate, AgNO3 to form yellow precipitate. In vinyl iodide, there is conjugate effect and in phenyl iodide, there is resonance effect which increases the C-I bond strength.
