Question
Question: Which of the following does not show mutaRotation? (A)  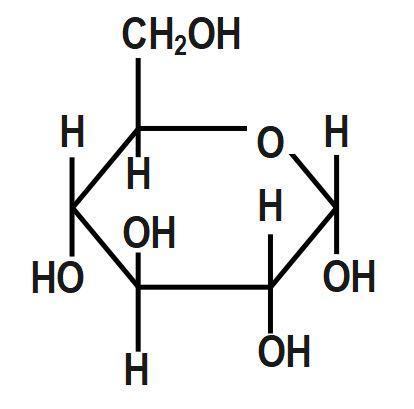
(B) 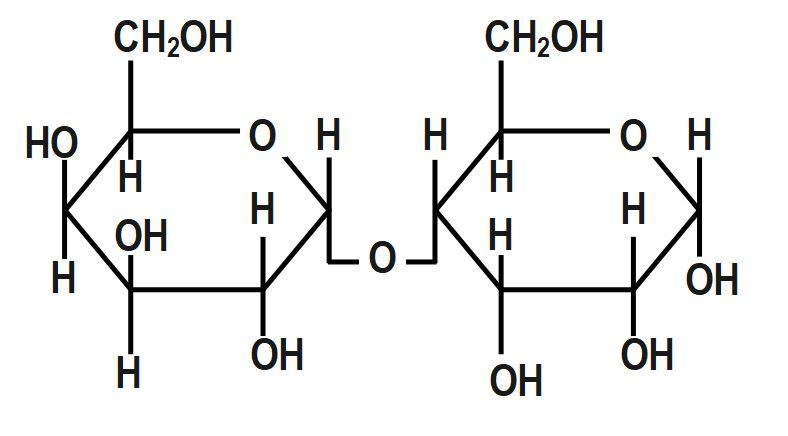
(C) 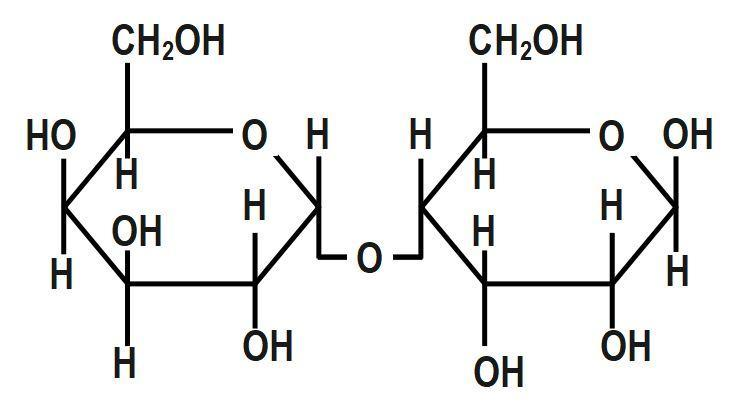
(D)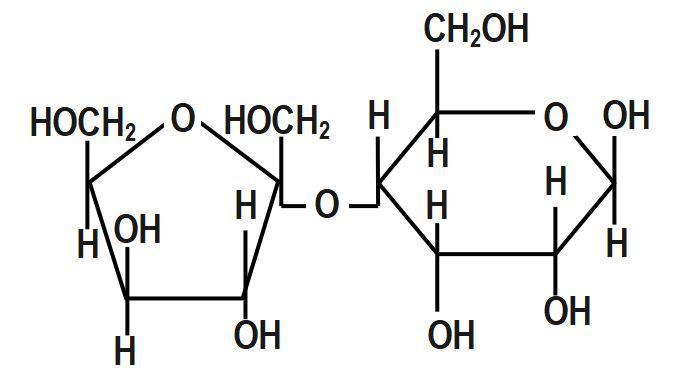
Solution
Hint : We know that for the compound to show mutarotation, presence of a hemiacetal linkage is an essential requirement. For a hemiacetal linkage, a six membered ring with a hydroxyl group is needed. Mutarotation is the change in optical rotation because of the change in equilibrium of the two anomers, when corresponding stereocenters interconvert.
Complete Step By Step Answer:
First, we have to understand that mutarotation is the process in which the optical rotation of the compound changes in the aqueous solution. This is due to the difference in the equipoise between two anomers. Now we shall discuss the structure of the given options in detail. The anomeric carbon is the carbon where the ring forms between the hydroxyl carbon and the carbonyl carbon. Without this hydroxyl group, the ring cannot open and close and therefore not undergo mutarotation. Glucose, fructose, maltose as well as galactose all have a free hydroxyl group and thus are known as reducing sugars. Hence, all of these will undergo mutarotation.
A hemiacetal forms when an aldehyde reacts with an alcohol. We also know that cellulose does not undergo mutarotation like sucrose. At the anomeric location of cellulose, they do not have hydroxyl group availability. As a result, cellulose does not undergo mutarotation. When glucose is in cyclic form, we can see hemiacetal linkage but it is not seen in a straight chain of glucose. Under acidic conditions the hemiacetal form of glucose can react with other alcohols to give acetals known as glycosides. These are widely distributed in nature. Non-reducing sugars such as sucrose cannot undergo mutarotation. This is because the glycosidic bond that forms between glucose and fructose in sucrose eliminates the availability of hydroxyl groups. Thus only option B will not show mutarotation:
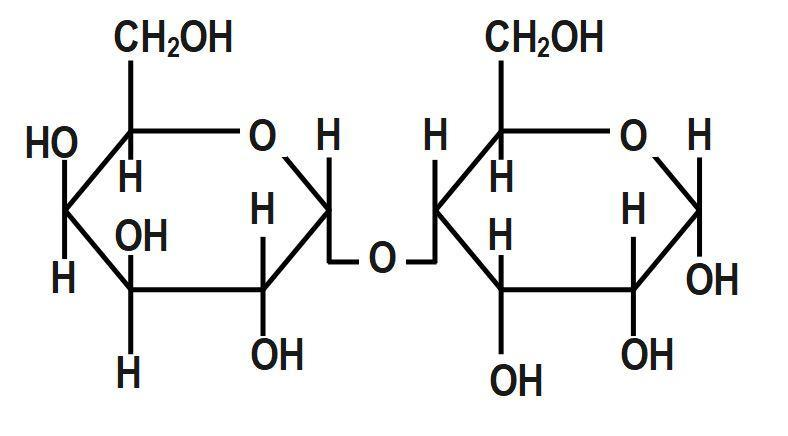
Therefore, the correct answer is option B.
Note :
Remember that sigma radicals do not have a stabilization effect hence they are more reactive. Free radicals are used in the manufacturing of various polymers. Polyvinyl chloride is prepared by the polymerization reaction following a free radical mechanism that initiates the chain reaction. Alkoxy free radical initiates the polymerization reaction.
