Question
Question: Which of the following does not show an Iodoform test? (a)- Ethyl methyl ketone (b)- Isopropyl k...
Which of the following does not show an Iodoform test?
(a)- Ethyl methyl ketone
(b)- Isopropyl ketone
(c)- 3-Methyl-2-butanone
(d)- Isobutyl alcohol
Solution
Draw the structure of the compounds and if the compound has a methyl ketone group or primary alcohol group then it will give the Iodoform test. Methyl ketone group is CH3−CO− and the primary alcohol group is CH3−CH(OH)−.
Complete answer:
Iodoform is a test that is used to distinguish between the compounds having either methyl ketone group or primary alcohol group. In this test, the compound having the group is treated with iodine and sodium hydroxide will form the Iodoform (CHI3).
Let us check all the compounds given in the option:
(a)- Ethyl methyl ketone
In this compound, the ketone group is attached with the ethyl group on one side and the methyl group on the other side. So the structure of the compound is:
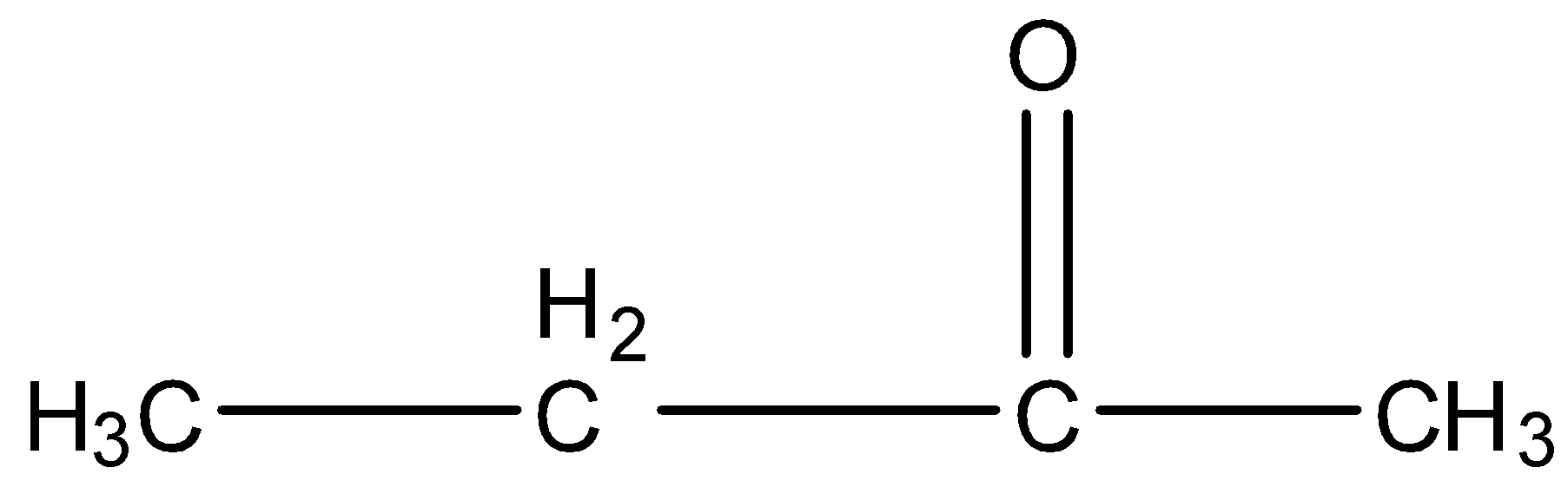
In this compound there is a methyl ketone group, as shown:
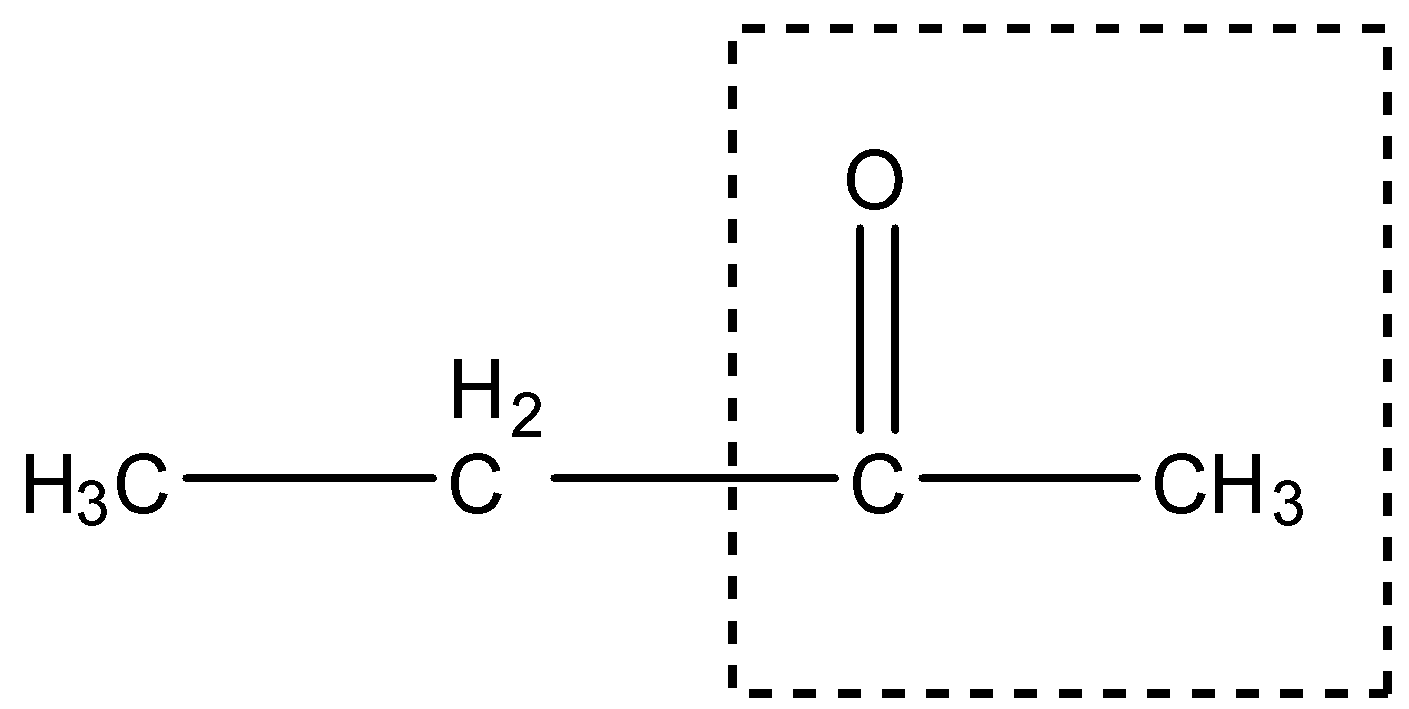
Therefore, it will give the Iodoform test.
(b)- Isopropyl alcohol
This is a three-carbon atom compound in which the second carbon atom has the alcohol group. The structure of the compound is given below:
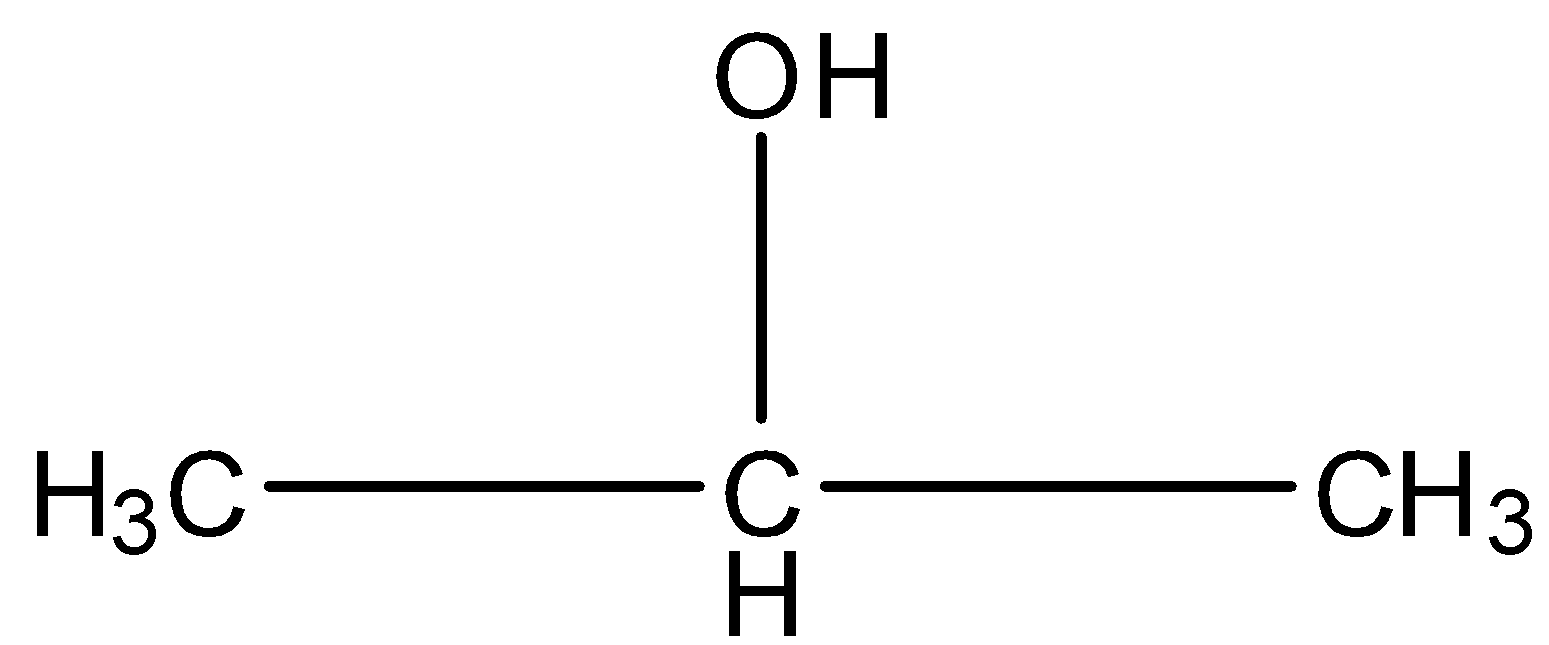
This compound has a primary alcohol group as shown:
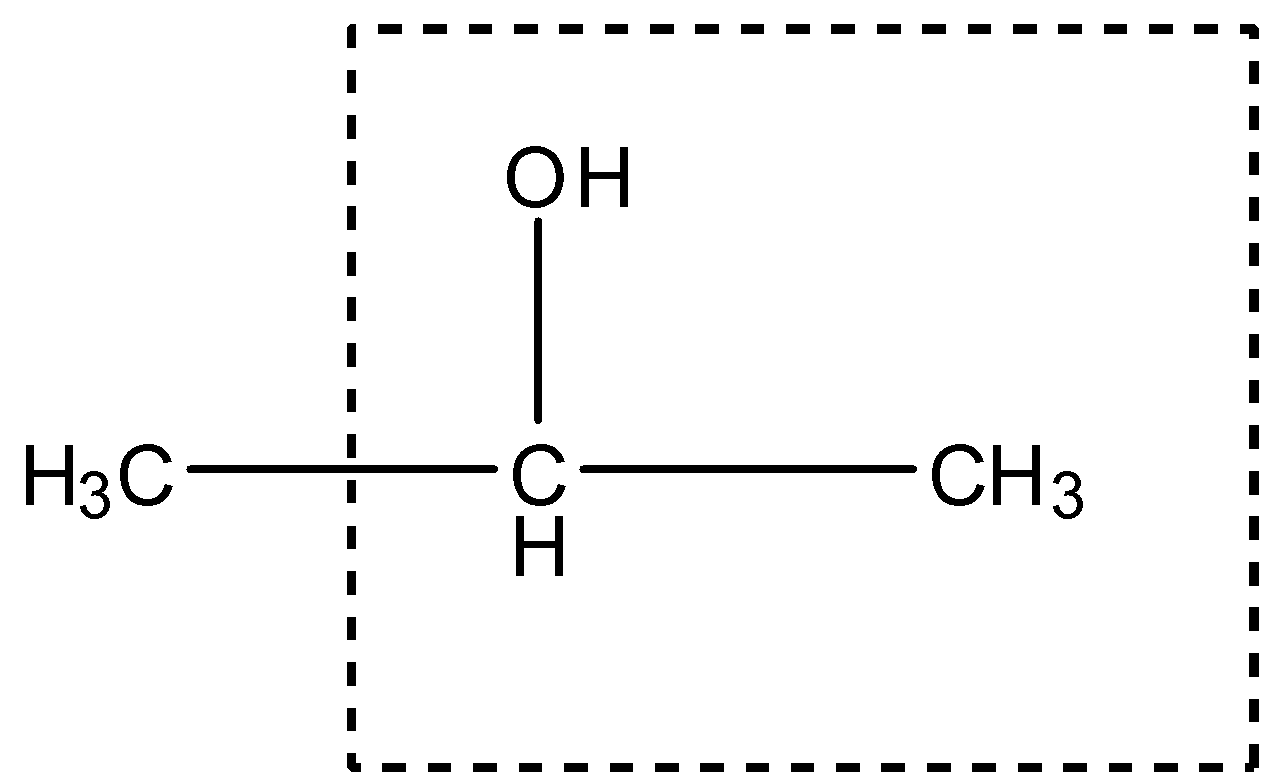
Therefore, it will give the Iodoform test.
(c)- 3-Methyl-2-butanone
This contains five carbon atoms in which the chain is of four carbon atoms, the second is the ketone group and the third carbon atom contains the methyl group. The structure is given below:
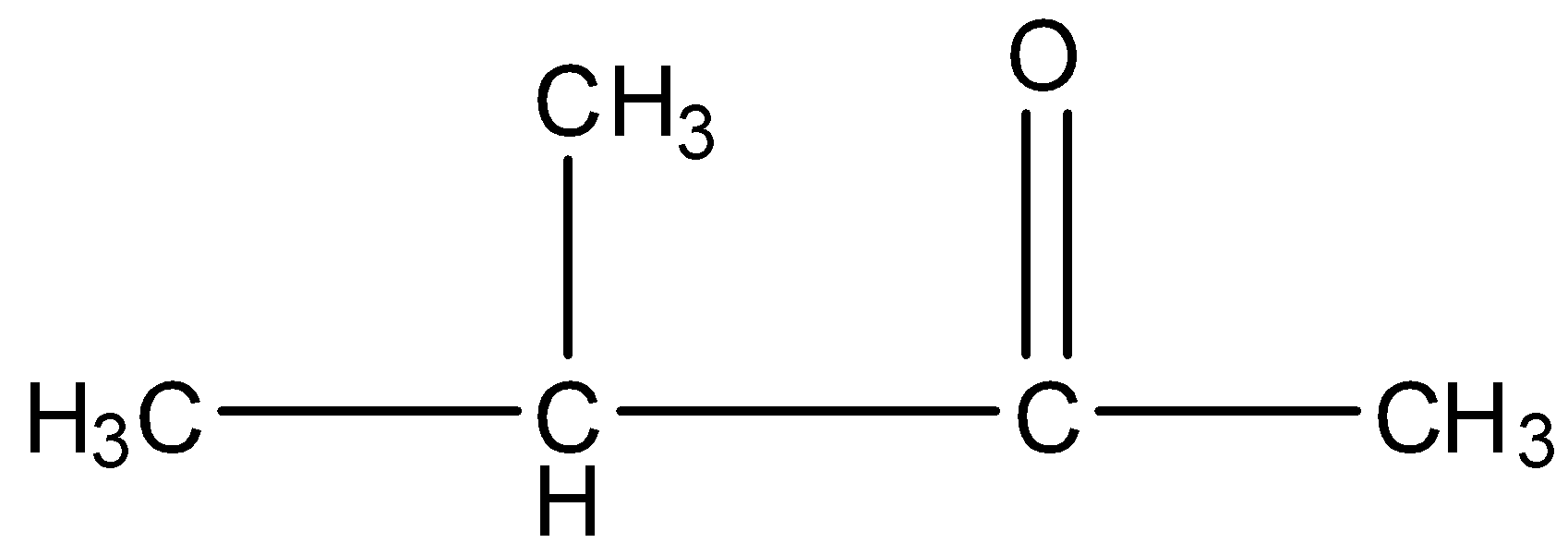
In this compound there is a methyl ketone group, as shown:
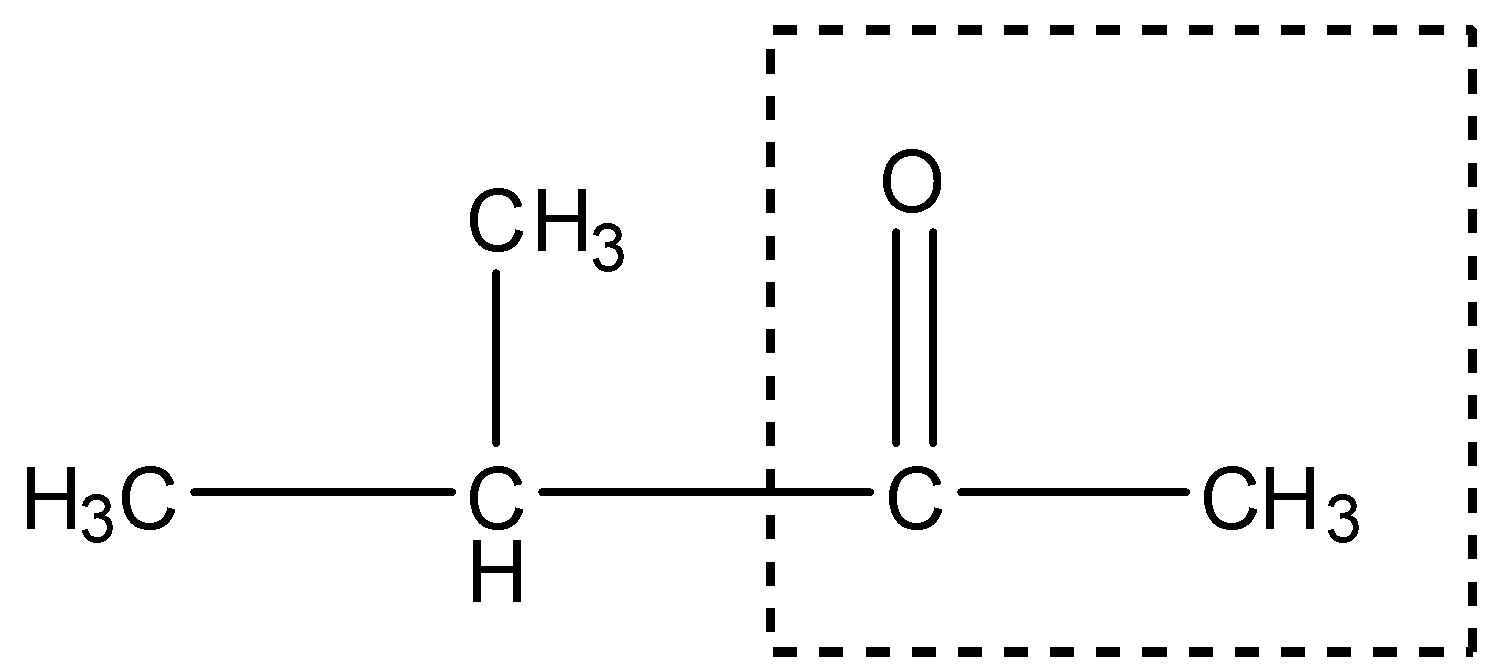
Therefore, it will give the Iodoform test.
(d)- Isobutyl alcohol
In this compound, there is a chain of three carbon atoms and one methyl group is present as a substituent group. The structure is given below:
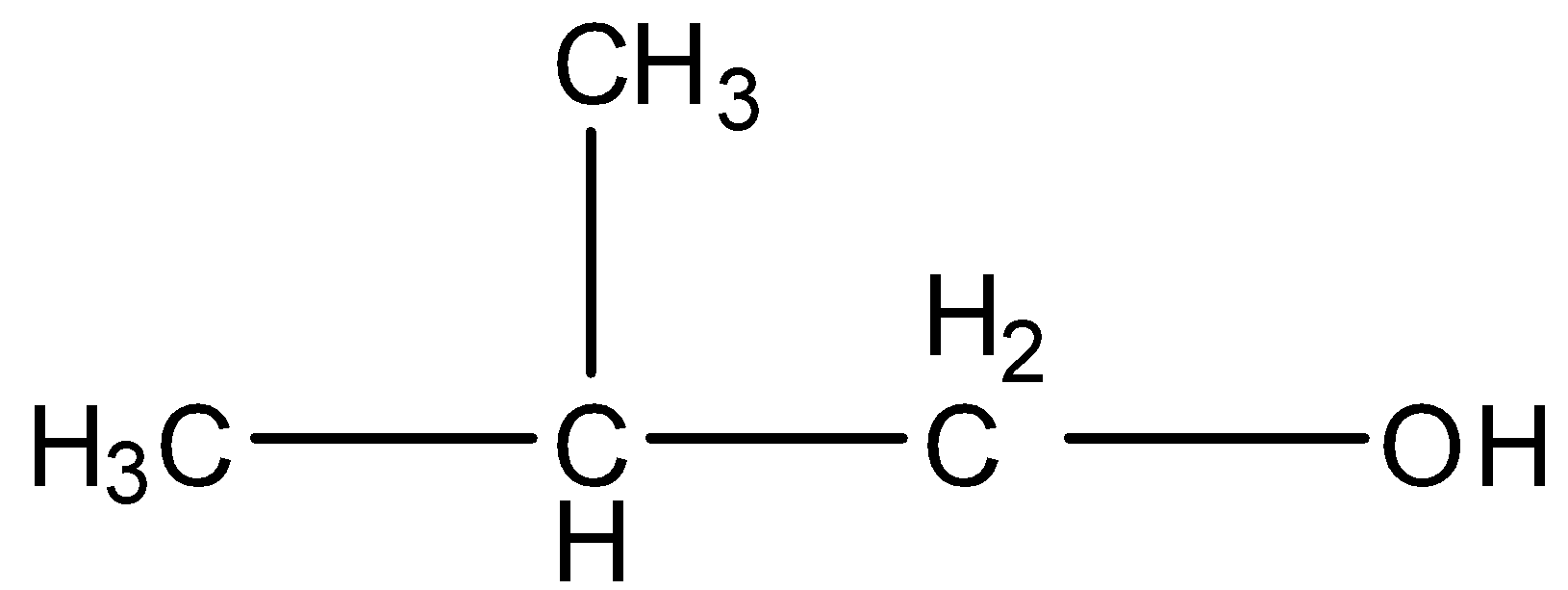
In this compound, there is no methyl ketone group and primary alcohol group, so this compound will not give the Iodoform test.
Therefore, the correct answer is an option (d).
Note:
In the iodoform test, the medium should be basic so sodium hydroxide is used. The Iodoform in the reaction is collected as the yellow precipitate in the solution indicating the completion of the reaction.
