Question
Question: Which of the following does not have \({{s}}{{{p}}^2}\) hybridization? A. \({{C}}{ _6}{{{H}}_6}\) ...
Which of the following does not have sp2 hybridization?
A. C6H6
B. C2H4
C. BCl3
D. C2H2
Solution
Let’s know about hybridization first. When we combine two or more atomic orbitals having similar energies, we get a new form of orbitals which are called hybrid orbitals. This phenomena is called hybridization.
Complete step by step answer:
We know that compounds having double bonds will have sp2 hybridization. Double bond indicates that they will have a σ bond and π bond. While single bonded compounds have sp3 hybridization. Single bond indicates that it has only σ bond. Triple bonded compounds have sp hybridization. It has one σ bond and two π bonds. σ bond can be formed independent of any other bond between two atoms. While π bond can be formed only if there is a σ bond already formed.
Now let’s focus on the structures of these compounds to know more about hybridization.
A. The structure of C6H6 is given below:
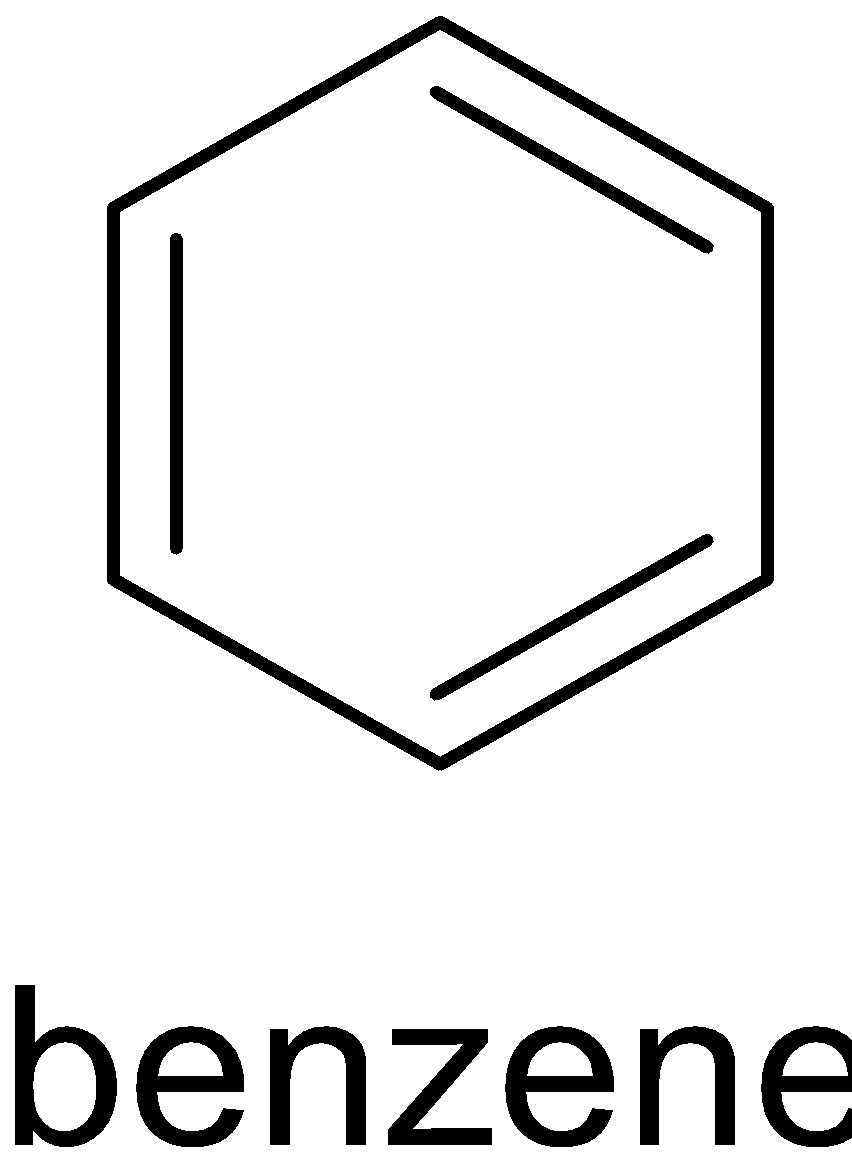
It has three π bonds. Thus all of the carbon atoms are sp2 hybridized.
B. The structure of C2H4 is given below:
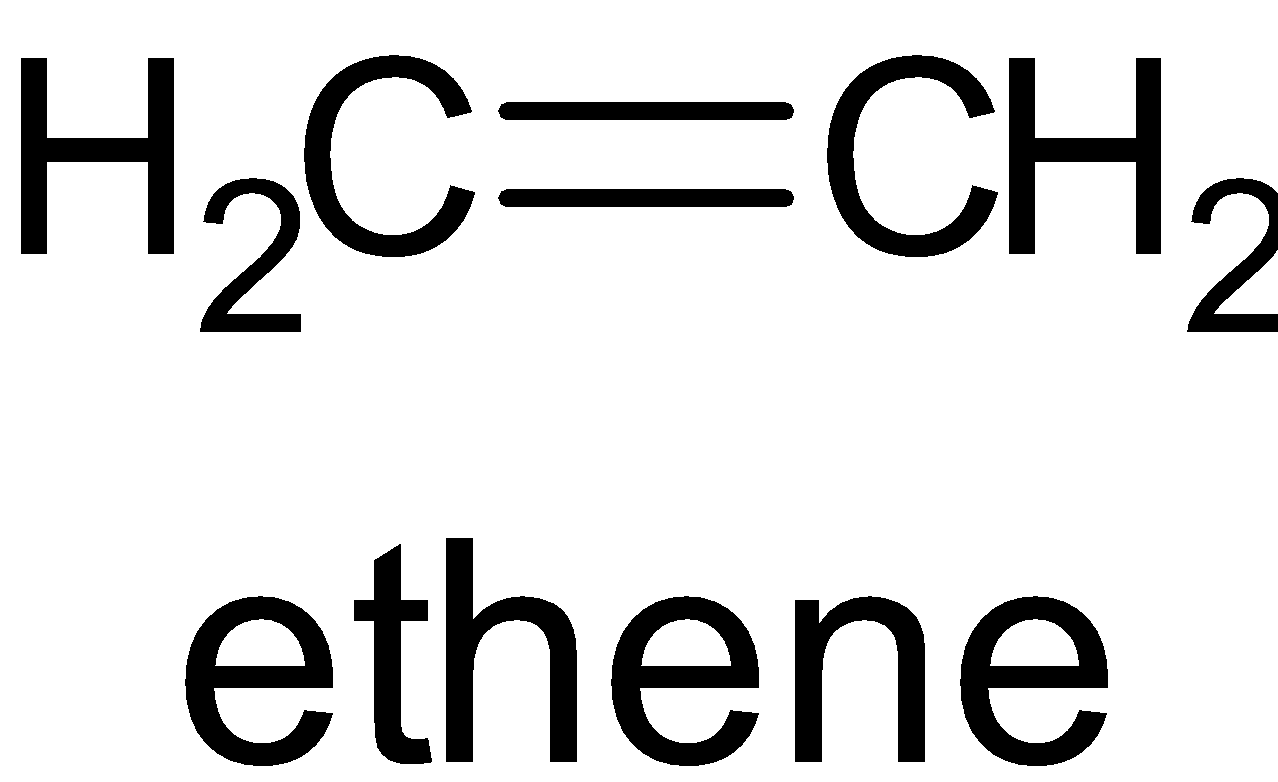
In this molecule, there is one σ bond and π bond. Thus both of the carbon atoms are sp2 hybridized.
C. The structure is given below:
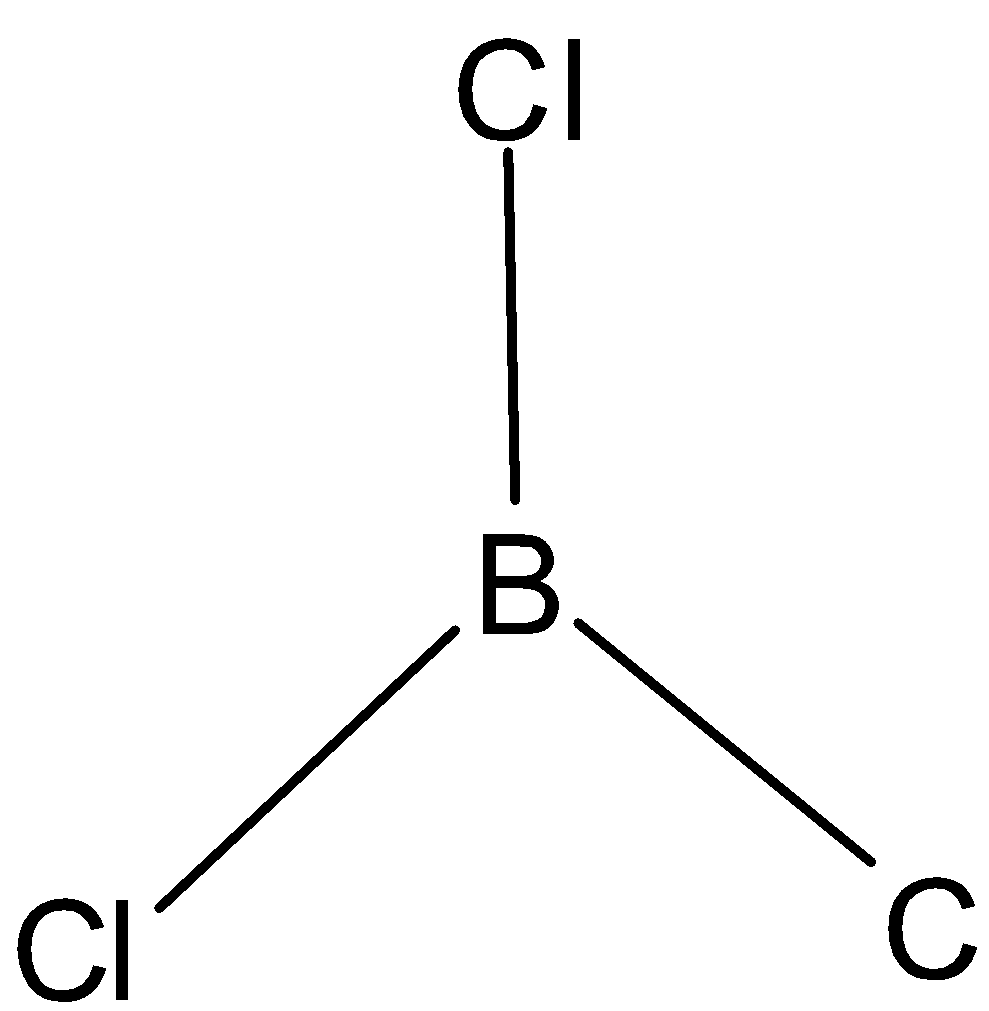
The hybridization in this molecule is determined using steric formula. The central atom is boron. Three chlorine atoms are attached to boron atoms. Steric number is obtained when we add the number of atoms bonded to the central atom and lone pairs. Here, there are no lone pairs. Thus steric number is three. Thus it has sp2 hybridization.
D. The structure is given below:
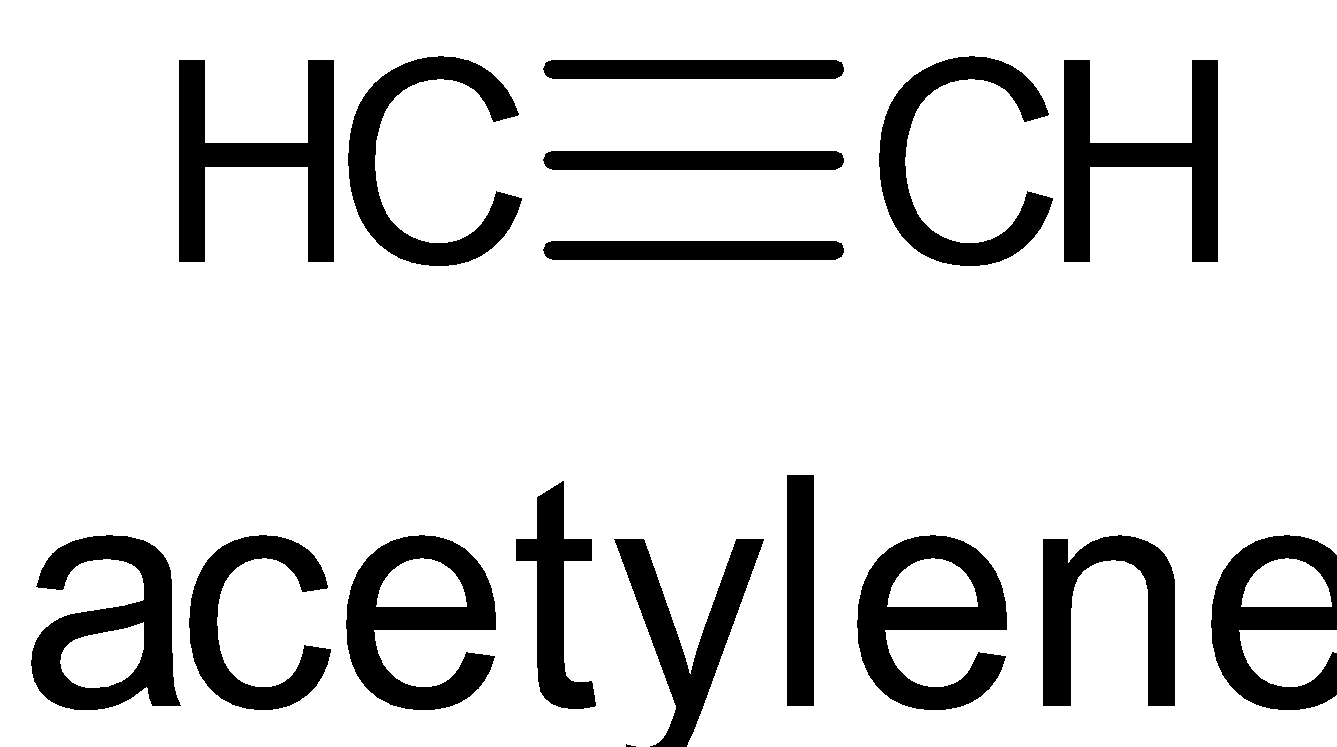
It has one σ bond and two π bonds. It has sp hybridization.
So BCl3 and C2H2 does not have sp hybridization.
So, the correct answer is Option D.
Note: Molecular geometry can be determined from the hybridization. Steric number shows the arrangement of electron pairs thereby we obtain the geometry. This attains a geometry so that the distance between the electron pairs are maximum. This is based on the VSEPR theory, i.e. valence shell electron pair repulsion theory.
