Question
Question: Which of the following does not apply to metallic bonds? (a)- Overlapping Valence Orbitals (b)- ...
Which of the following does not apply to metallic bonds?
(a)- Overlapping Valence Orbitals
(b)- Mobile Valence Electron
(c)- Delocalized Electron
(d)- Highly Directed Bond
Solution
Metallic bond is formed by the kernels of the metal or ions of the metal and electrons surrounding them. The electrons are surrounded by the kernel in all directions and the electrons surrounding the metal ions.
Complete answer:
First, let us see what metallic bond is formed. So, the metallic bond is formed between kernels of the metal and the delocalized electrons surrounding the kernels. Kernels of the metal are also known as metal ions. This is shown below:
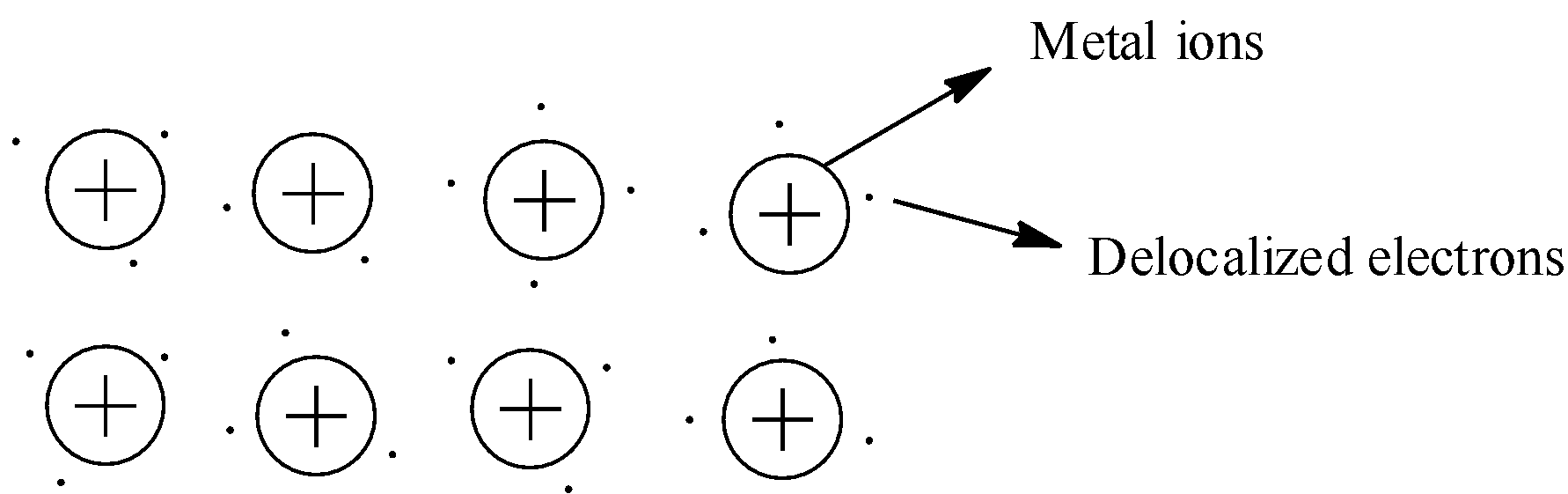
So, the electrons surrounding the kernels are in all directions. These electrons and metal ions have electrostatic forces. Since the electrons are surrounded in all the directions to the kernel, the electrostatic forces are in all the directions of the metal ion.
So, there is overlapping of the valence orbitals because without overlapping of orbitals no bond can form.
Since the electrons are delocalized, therefore, the electrons can be moved from one place to another. Since the force is in all directions we can say that the bond is not directed in any direction.
Therefore, from all the options (a), (b), and (c) can be true for the metallic bond.
Hence, the correct answer is option (d)- Highly Directed Bond.
Note:
Some of the bonds which have directions or which are called directional bonds are covalent bonds, coordinate bonds, etc. These are formed by the directions of overlapping of orbitals, movement of electrons, etc.
