Question
Question: Which of the following do not exhibit geometrical isomerism (Cis-trans): A.:
A.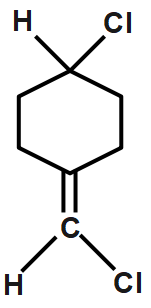
B.CH3CH=CCl2
C.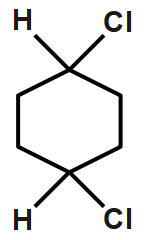
D.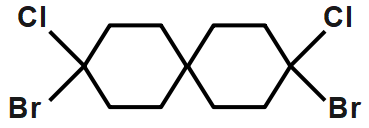
Solution
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
Complete step by step answer:
The word isomer is derived from the Greek words iso and mer which refers to the equal parts. There are two primary types of isomerism known as structural isomerism and stereoisomerism. Structural isomerism is commonly defined as constitutional isomerism in which the functional groups and the atoms in the molecules are linked in different orientations of the atoms belonging to the molecule in three-dimensional space.
Geometric isomers are the type of stereoisomerism it can also be known by the cis-trans isomers and this isomers have different spatial arrangements of atoms in three-dimensional space. To see which one will give a geometrical isomer first write the structure of every option.
This can show cis and trans isomer so we can say that it shows geometrical isomerism.
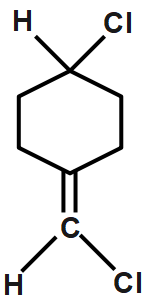
Cis and trans not possible since as chlorine atoms on the same carbon. Hence it does not have any cis or transform. Therefore, it does not exhibit geometrical isomerism. CH3CH=CCl2
This can show cis and trans isomer so we can say that it shows geometrical isomerism.
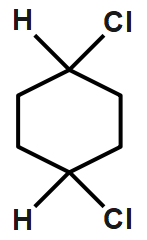
This can show cis and trans isomer so we can say that it shows geometrical isomerism.
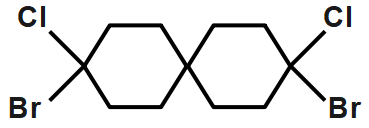
Hence, the correct option is B
Note: Cis isomers are molecules with the same connectivity of atoms they have similar side groups placed on the same side of a double bond while Trans isomers have molecules with similar side groups placed on opposite sides of a double bond.
