Question
Question: Which of the following describes the bonds in \(PC{l_5}\)? A. 2 axial and 3 equatorial B. 3 axia...
Which of the following describes the bonds in PCl5?
A. 2 axial and 3 equatorial
B. 3 axial and 2 equatorial
C. 4 axial and 5 equatorial
D. 5 axial and 4 equatorial
Solution
We have to first predict the geometry of the compound to describe the bonds. We can use VSEPR theory to predict the geometry of the compound. We have to first predict the steric number using the bond pairs that are bonded to the central atom and the number of lone pairs present in the central atom.
Complete answer:
In PCl5, five atoms of chlorine are bonded to one atom of phosphorus. Let us now draw the Lewis structure of PCl5. In order to draw the Lewis structure, we first need to calculate the total number of valence electrons in PCl5.
The total number of valence electrons in phosphorus is five and for five chlorine atoms, the total number of valence electrons is thirty-five. So, the total number of valence electrons in PCl5 is forty electrons. We can draw the Lewis structure as,
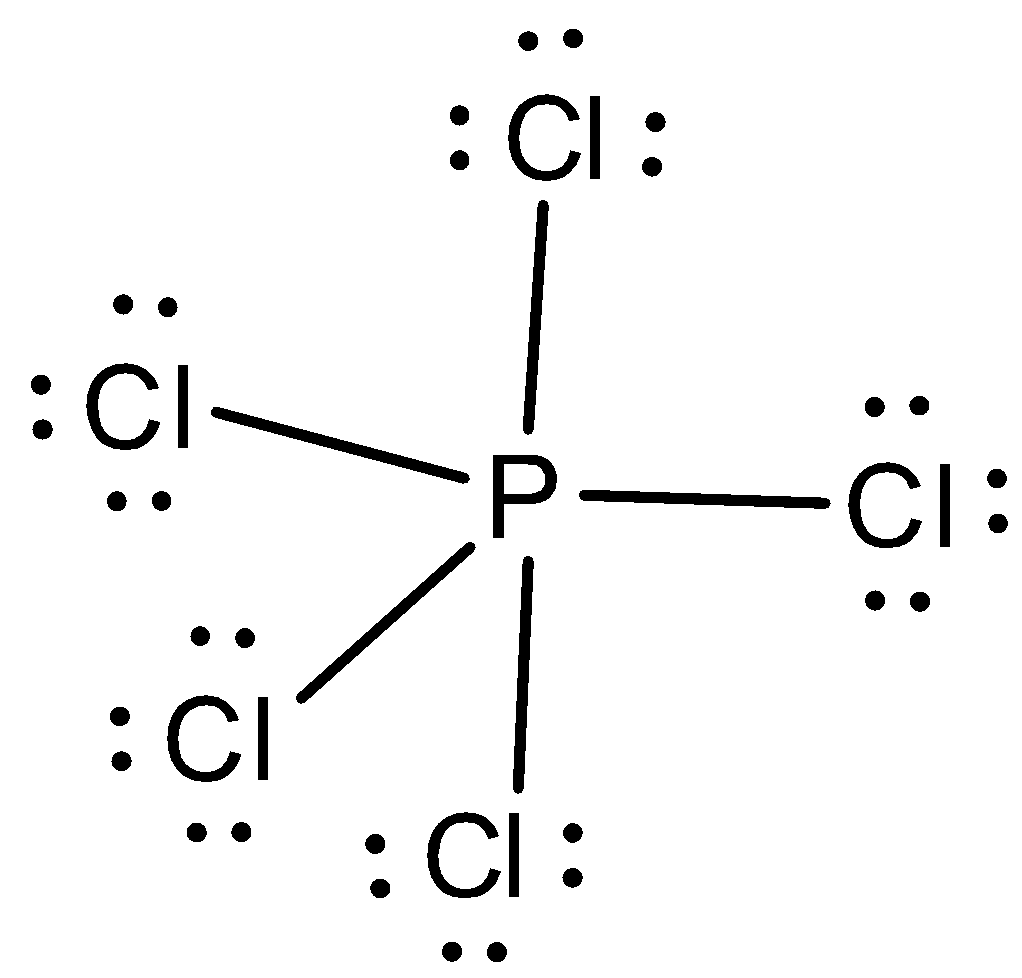
Let us now predict the steric number.
From the Lewis structure, we can see that the central atom has no lone and there are five bond pairs in the central atom. So, the steric number is five. When the steric number is five with no lone pairs in the central atom, the hybridization is sp3d and the molecular geometry is trigonal bipyramidal and the electron pair geometry is also trigonal bipyramidal. The bond angles are 90∘,120∘. There are three equatorial bonds and two axial bonds.
So, the correct answer is “Option A”.
Note:
We have to know that in PCl5, the axial bonds are longer when compared to equatorial bonds because of the greater repulsion from equatorial bonds. Hence, axial bonds are closer to equatorial bonds and this leads to more repulsion leading to bond length elongation.
