Question
Question: Which of the following describes the best relationship between the methyl groups in the chair confor...
Which of the following describes the best relationship between the methyl groups in the chair conformation of the substance shown below?
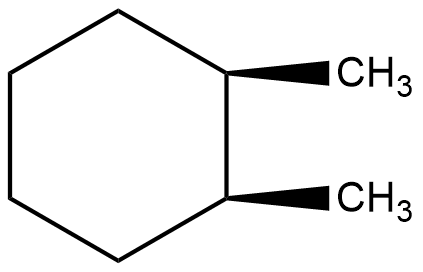
A. Trans
B. Anti
C. Gauche
D. Eclipsed
Solution
Generally, six membered cyclic organic compounds are going to show high stability in their chair confirmation form. The substituents on the carbon atoms are going to exit in axial or equatorial positions.
Complete step by step answer:
- In the question it is asked to find the best relationship between the methyl groups in the chair conformation of the given compound.
- The Chair form of 1,2-dimethyl cyclohexane is as follows.
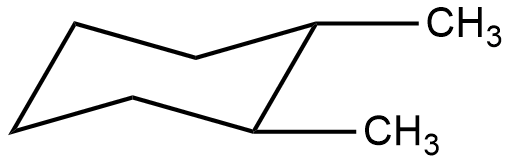
- In the chair form the two methyl groups are cis to each other as per the above structure.
- We can write the conformation for the 1,2-dimethyl cyclohexane and by using the confirmation we can find the relationship between the methyl groups in the chair conformation of the given compound.
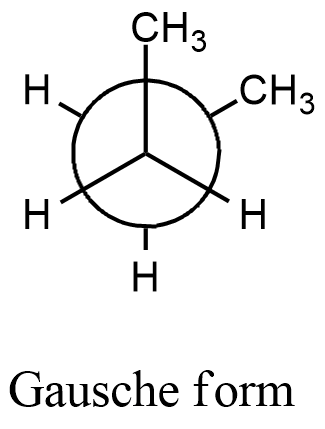
- From the above confirmation we can say that the two methyl groups are going to have an angle of 60 degrees.
- In eclipsed conformation of 1,2-dimethyl cyclohexane the methyl groups are going to overlap one on another.
- Means the gauche form is a more stable conformation for 1,2-dimethyl cyclohexane.
So, the correct option is C.
Note:
In confirmations of the molecules always the gauche form is more stable than the remaining staggered and eclipsed conformations. The reason behind the high stability of the gauche form is due to the less torsional angle between the bulky groups.
