Question
Question: Which of the following defect(s) is seen in FeO? (A) Metal deficiency defect (B) Metal excess de...
Which of the following defect(s) is seen in FeO?
(A) Metal deficiency defect
(B) Metal excess defect
(C) Displacement defect
(D) Impurity defect
Solution
It shows a non-stoichiometric defect. Most of the non-stoichiometric defects are shown by the transition metals. However, they also include halides, hydrides, carbides and nitrides. The simplest way to classify non-stoichiometric compounds is to consider which element is in excess or deficient and how this excess is brought about.
Complete step by step solution:
-As we know, solids are formed by a large network of crystals and this network is not always perfect. There are defects in these crystals which are generally irregular in the arrangement of the constituents forming the network.
-We can classify these defects as point defects which is the irregularity from the ideal arrangement around a point or an atom and line defects which is the irregularity in the entire row of the lattice.
-Point defects are further classified into stoichiometry defects, impurity defects and non-stoichiometric defects.
-We can define the non-stoichiometry defects as the defects which contain elements in a non-stoichiometric ratio due to excess or deficiency of metal in the lattice.
-Those defects which disturb the stoichiometry of the compounds are called non-stoichiometry defects. These defects are of two types:
-Metal excess defect
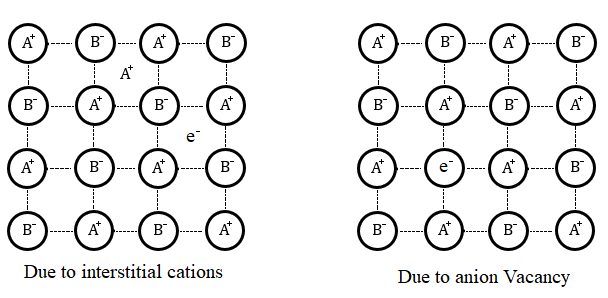
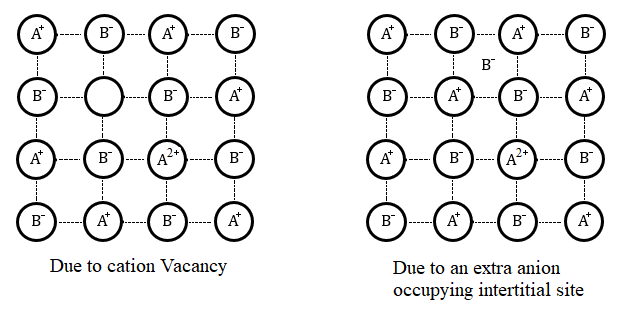
-Metal deficiency defect.
Metal excess defects occur due to anionic vacancies. Mostly, alkyl halides show this type of defect. When a crystal of NaCl is heated in the presence of sodium vapour, the atoms of sodium are deposited on the surface of the crystal and the chlorine anion diffuses to the surface of the crystal and gives us NaCl. This happens by the loss of electrons by sodium atoms, the electrons released occupy the anionic site and as a result of the crystal now have an excess of sodium.
However, iron oxide, FeO shows an example of metal deficiency defect. It is mostly found in the composition of Fe0.95O . It can range from Fe0.94O to Fe0.96O.
The Fe2+ cations are missing from the crystals of FeO and the loss of the positive charge is made up by the Fe3+ ions.
Therefore, we can say that the correct answer is option (A) metal deficiency effect.
Note: In the metal excess defect when the anionic sites are occupied by the unpaired electrons, they are called F-centres (Farbenzenter centres) due to these centres the metal imparts specific colours, the colour results due to the excitation of electrons when they absorb energy from the visible light falling on the crystal.
