Question
Question: Which of the following curve correctly represents \({{\text{S}}_{\text{N}}}\text{1}\) and \({{\text{...
Which of the following curve correctly represents SN1 and SN2 ?
A. 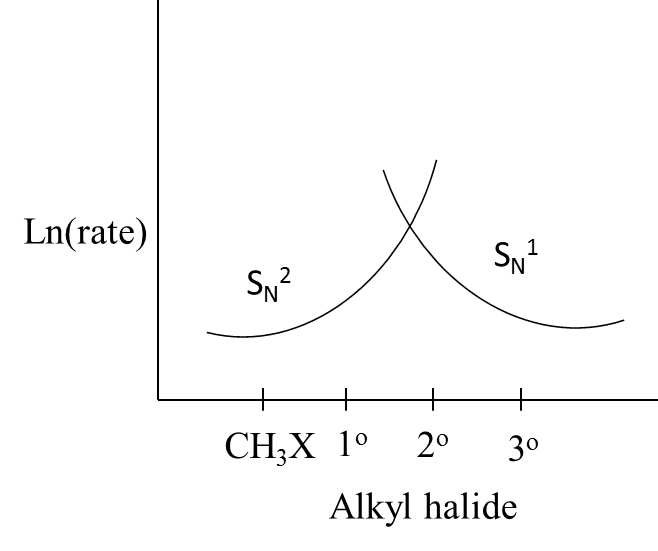
B. 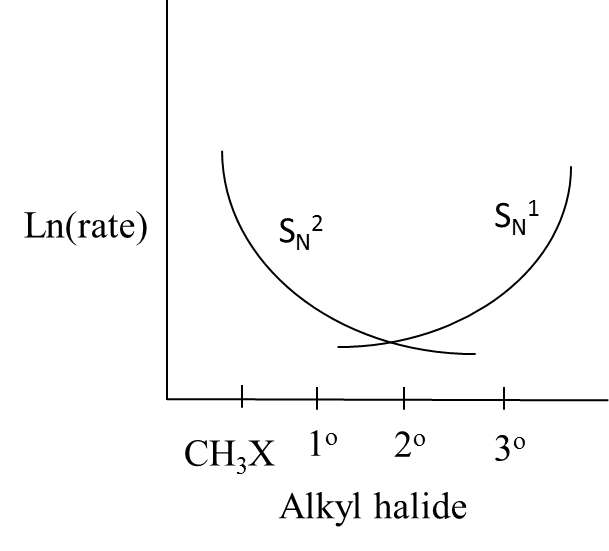
Solution
SN means nucleophilic substitution reaction. Nucleophilic substitution reactions are two types. They are SN1 and SN2 . SN1 is called unimolecular substitution reaction and SN2 is called bi-molecular nucleophilic substitution reaction.
Complete step by step answer:
- In the question it is given that which curves correctly explains the SN1 and SN2 reactions.
- We know that in unimolecular nucleophilic substitution reactions the rate of the reaction is going to depend on the concentration of the one molecule only.
- But in the case of a bi-molecular nucleophilic substitution reaction, the rate of the reaction is going to depend on the concentration of the two reactants which are involved in the reaction.
- We can say that the rate of the SN1 is high when the reactant contains tertiary alkyl halides.
- The order of increasing the rate of SN1 reaction in case of alkyl halides is as follows.
CH3X<1o<2o<3o
- Means the SN1 reaction occurs easily in tertiary alkyl halides compared to simple and primary alkyl halides.
- Coming to the case of SN2 reaction, the rate of the SN2 is high when the reactant contains primary alkyl halides.
- We can say that the rate of the SN2 is high when the reactant contains simple and primary alkyl halides as the reactant.
- The order of decreasing the rate of SN2 reaction in case of alkyl halides is as follows
CH3X>1o>2o>3o
- Therefore we can say that the curves in option B exactly suit the above explanation.
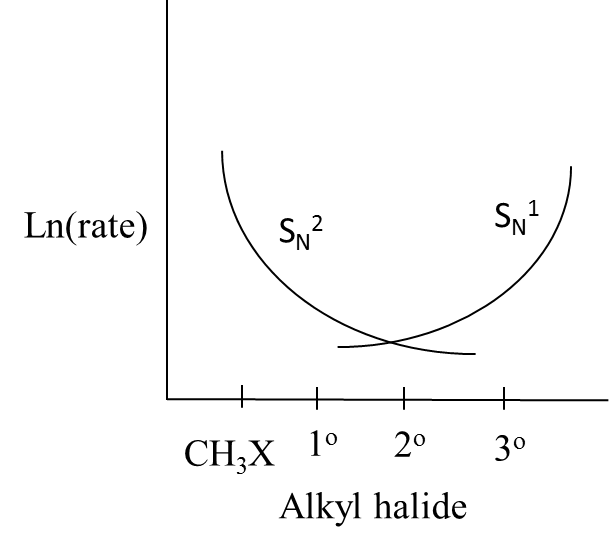
So, the correct answer is “Option B”.
Note: The rate of the nucleophilic substitution reactions depends on the type of alkyl halides involved in the reaction. The reaction is going to be initiated by the nucleophile that is why the reaction is called nucleophilic substitution reaction.
