Question
Question: Which of the following correctly ranks the aryl halides in increasing order of reactivity towards so...
Which of the following correctly ranks the aryl halides in increasing order of reactivity towards sodium methoxide in methanol?
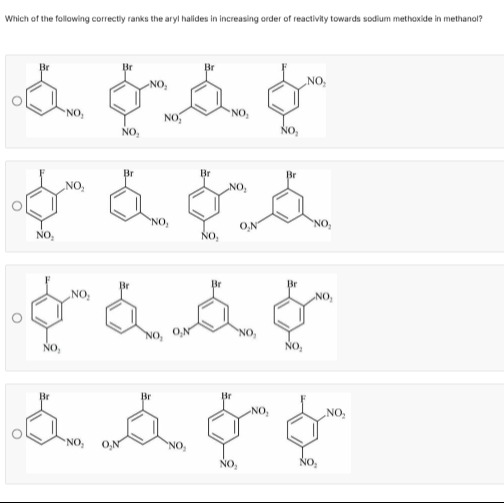
c1c(Br)cc(N(=O)=O)cc1 < c1c(F)c(N(=O)=O)cc(N(=O)=O)c1 < c1cc(Br)cc(N(=O)=O)c1N(=O)=O < c1c(Br)c(N(=O)=O)cc(N(=O)=O)c1
c1cc(Br)cc(N(=O)=O)c1 < c1cc(Br)cc(N(=O)=O)c1N(=O)=O < c1c(F)c(N(=O)=O)cc(N(=O)=O)c1 < c1c(Br)c(N(=O)=O)cc(N(=O)=O)c1
c1c(F)c(N(=O)=O)cc(N(=O)=O)c1 < c1c(Br)c(N(=O)=O)cc(N(=O)=O)c1 < c1cc(Br)cc(N(=O)=O)c1N(=O)=O < c1cc(Br)cc(N(=O)=O)c1
c1cc(Br)cc(N(=O)=O)c1 < c1cc(Br)cc(N(=O)=O)c1N(=O)=O < c1c(Br)c(N(=O)=O)cc(N(=O)=O)c1 < c1c(F)c(N(=O)=O)cc(N(=O)=O)c1
c1cc(Br)cc(N(=O)=O)c1 < c1cc(Br)cc(N(=O)=O)c1N(=O)=O < c1c(Br)c(N(=O)=O)cc(N(=O)=O)c1 < c1c(F)c(N(=O)=O)cc(N(=O)=O)c1
Solution
The reaction of aryl halides with sodium methoxide in methanol is a Nucleophilic Aromatic Substitution (SNAr) reaction, specifically an addition-elimination mechanism. The reactivity of aryl halides in SNAr reactions is primarily governed by:
-
Electron-withdrawing groups (EWGs): Strong EWGs, particularly at the ortho and para positions relative to the leaving group, stabilize the negative charge in the intermediate Meisenheimer complex. Nitro groups (-NO₂) are strong EWGs. Meta-positioned EWGs only exert an inductive effect, which is less effective.
-
Nature of the leaving group: For SNAr reactions, the leaving group ability generally follows the order F > Cl > Br > I. The strong inductive effect of fluorine effectively stabilizes the negative charge, facilitating the attack.
Based on these factors, the correct order is:
Compound 1 (One meta -NO₂) < Compound 3 (Two meta -NO₂) < Compound 2 (Two ortho/para -NO₂) < Compound 4 (Two ortho/para -NO₂ with F)
