Question
Question: Which of the following contains three centre and two electron bonds? (A)- \({\left( {{\text{Be}}{{...
Which of the following contains three centre and two electron bonds?
(A)- (BeH2)2
(B)- LiAlH4
(C)- (BeCl2)2
(D)- Li2C2
Solution
As the name suggests, ‘three centre and two electron bonds’ means we have to choose that compound in which 3 centers is presented as atoms and 2 bonds is present in the given compound.
Complete step by step solution: Three centre and two electron bonds containing compounds are generally electron deficient in nature because 2 electrons are shared in between three atoms.
Among the given options in the question option (A) i.e. (BeH2)2is correct, below is given some important points which throw light on the reason:
- Atomic no. of Beryllium (Be) atoms is 4 and its electronic configuration is1s22s2 .
- There are 2 electrons in the valence shell, it means we use these 2 electrons for the formation of bonds.
- Among these 2 electrons, one electron forms a covalent bond with one hydrogen atom and another electron is used to form a banana bond with another hydrogen atom.
- In the given compound(BeH2)2, total no. of Be atom is 2 and total no. of hydrogen atom is 4; among which 2 hydrogen forms covalent bond & another 2 takes part in the formation of banana bond.
- One Banana bond is formed by the 2 electrons between the 3 atoms but there is no uniform distribution of electrons among all 3 atoms, it is more towards the 2 atoms only.
- The structure of (BeH2)2is shown as:
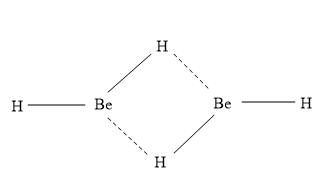
- Here Be has sp2 hybridization.
- Be-H-Be is a 3 centre between them 2 electron bonds are present, which is known as banana bond.
So, option A is the correct answer.
Note: In this question it is mentioned that between 3 centre, 2 electron bonds is present so never confuse with the Be-H bond because in this bond 2 centre is only present. And as Be forms 3 bonds but among these 3 bonds, electrons are not present in that bond which is shown by the dash line.
