Question
Question: Which of the following contains only three pairs of electrons? A. Carbocation B. Carbanion C. ...
Which of the following contains only three pairs of electrons?
A. Carbocation
B. Carbanion
C. Free radical
D. All of these
Solution
Whenever a single bond is formed between two atoms then this bond contains two electrons. We will analyze each given option with the help of suitable examples. Then we can find the number of bonds it makes and number of free electrons if available. Thus we can find the total number of pairs of electrons in each compound.
Complete Step By Step Answer:
The atoms which take part in covalent bond formation, each atom share an electron to form a single covalent bond. Thus we can say that whenever there is a bond present between any atoms, then this bond will contain two electrons. Now we will take suitable examples of each given option and then find the total number of electron pairs in each compound.
A. Carbocation
For example: Methyl Carbocation (CH3+). Its structural formula can be represented as:
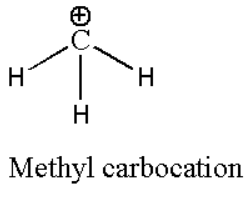
Since we know that each bond will contain two electrons, then it contains three bonds which means it contains (3 × 2) six electrons. Positive charge is due to loss of electrons by carbon atoms. Thus we can say that carbocation contains six electrons or three pairs of electrons.
B. For carbanion:
For example: Methyl carbanion (CH3−). Its structural formula can be represented as:
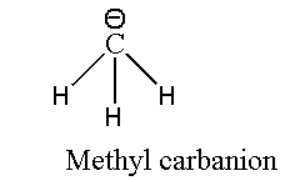
Similarly it forms three bonds but it has a negative charge on carbon atoms which means it has one more pair of electrons due to this negative charge. Therefore in total it contains four pairs of electrons.
C. Free Radical:
For example: Methyl radical (CH3∙). Its structural formula can be represented as:
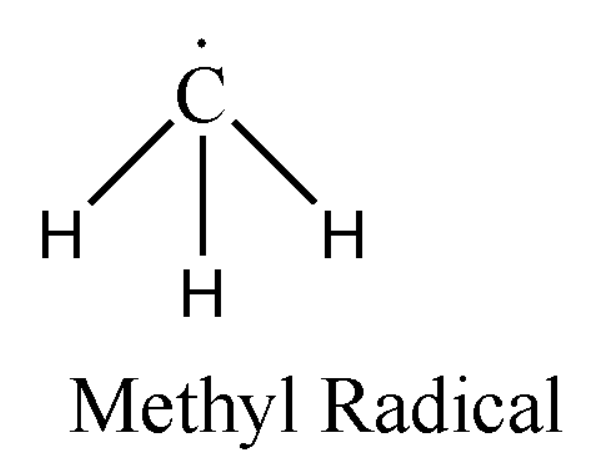
Here also it forms three bonds with hydrogen but it has got a free radical or single electron left on the carbon atom. Thus we can say that it contains seven electrons in total.
Thus on analyzing each compound we know that carbocation contains three pairs of electrons.
Hence the correct option is A.
Note:
Free radical is formed when there is homolytic cleavage of bond between the atoms. We can take other alkyl groups too but we take methyl compounds for easy calculation. The single covalent bond is also known as sigma bond. Thus sigma bonds contain two electrons respectively.
