Question
Question: Which of the following contains coordinate covalent bonds? A. \[{{\text{N}}_2}{{\text{H}}_5}^ + \]...
Which of the following contains coordinate covalent bonds?
A. N2H5+
B. BaCl2
C. HCl
D. H2O
Solution
Coordinate covalent bond is also known as dative covalent bond. This is a special type of covalent bond in which both the shared electrons are contributed by one atom only. Metal and non-metal usually have ionic bonds. Non-metal and nonmetal bonds with covalent or coordinate bonds.
Complete step by step answer:
Covalent bond is the bond formed by sharing electrons by the atoms. While coordination bond is the
Coordinate covalent bond can be defined as a covalent bond in which both electrons of the shared pair are contributed by one of the two atoms. The atom which donates the electron pair is called donor and the species is called nucleophiles. The atom which accepts them for bond formation is called acceptor and the species is called electrophiles.
Coordinate covalent bond is generally represented by an arrow.
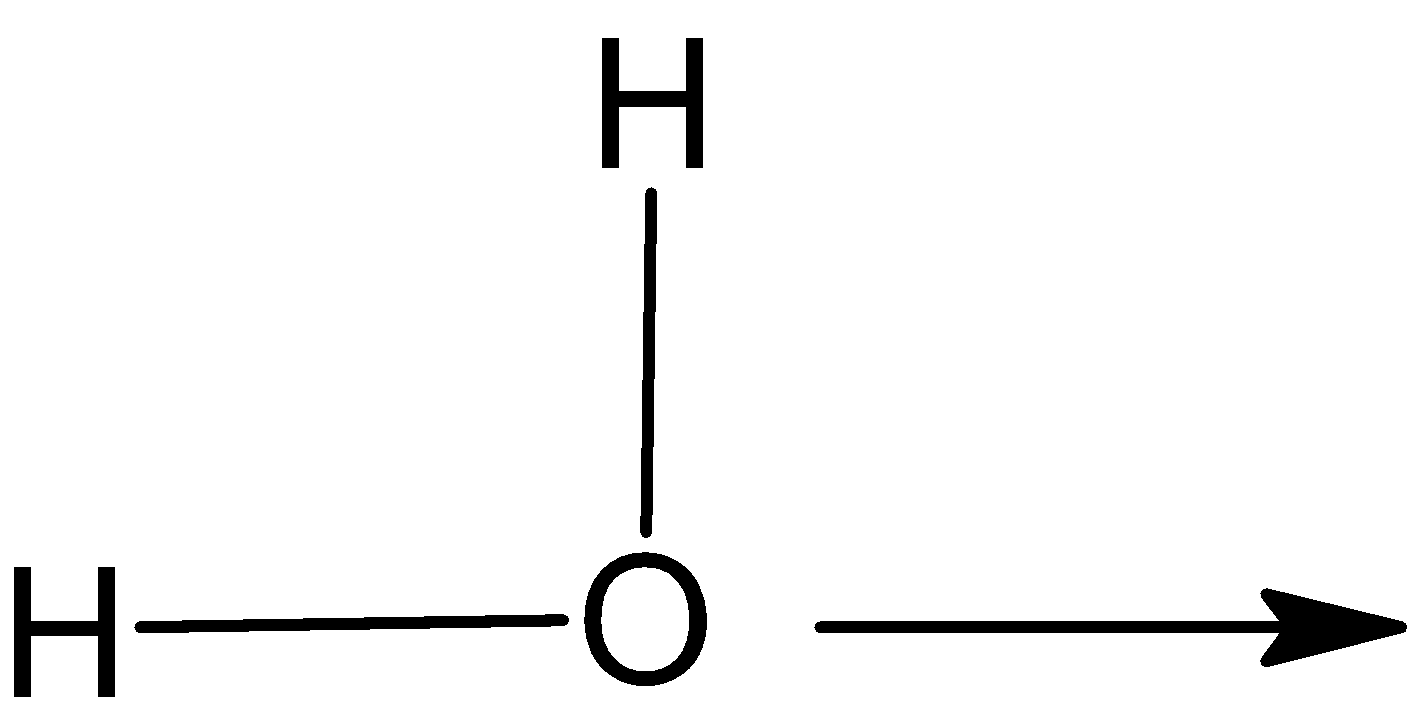 H
H
In this hydronium ion H3O+, head of arrow points toward the acceptor atom. The line is used to represent the covalent bond.
N2H5+ molecule is called hydrazinium molecule. It is a combination of N2H4 and H+. This is an example for a coordinate covalent bond. Coordinate bonds are formed from nitrogen to hydrogen. Each nitrogen has bonded to three hydrogen.
BaCl2 is an ionic bond while H2O and HCl are polar covalent bonds.
Hence, Option A is the correct option.
Additional information:
The compound formed via dative covalent bonding can be called an additional compound or adduct. They are very weak bonds. Similar to N2H5+, N2O5 is also a coordinate covalent bond.
Note:
A coordinate covalent bond is established between two atoms where:
One of the two atoms has a full outer shell and a nonbonding pair of electrons.
The other atom is one pair short of a full outer shell of electrons.
The dative bond once formed is indistinguishable from a regular covalent bond.
