Question
Question: Which of the following contains both polar and nonpolar bonds? (A) \[N{H_4}Cl\] (B) \(HCN\) (C...
Which of the following contains both polar and nonpolar bonds?
(A) NH4Cl
(B) HCN
(C) H2O2
(D) CH4
Solution
Due to difference in electronegativities of atoms, electrons are unequally shared between them and lead to formation of polar covalent bonds, whereas, If the electronegativities of two atoms is same, equal sharing of electrons occurs between them and forms non-polar covalent bond.
Complete step by step answer:
Considering each option one by one.
(A) NH4Cl (Ammonium chloride)
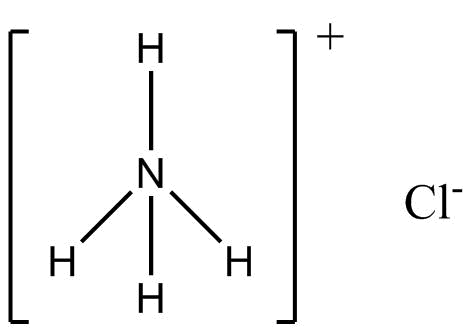
Nitrogen is more electronegative than hydrogen, therefore, nitrogen will acquire a partial negative charge because it will attract the shared pair of electrons towards itself. Hydrogen will acquire a partial positive charge. Hence, NH4Cl will be a polar compound with dipole moment towards nitrogen.
(B) HCN (Hydrogen Cyanide)
H−C≡N:
In this method, nitrogen acquires a partial negative charge and hydrogen acquires a partial positive charge because of difference in electronegativities. The dipole moment is towards nitrogen.
(C) H2O2 (Hydrogen peroxide)
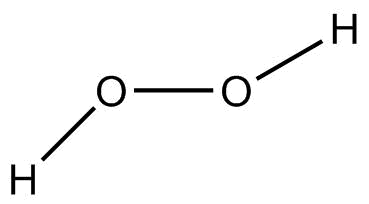
In H2O2, oxygen is more electronegative than hydrogen. Therefore, oxygen attracts the shared pair of electrons towards itself due to which oxygen develops partial negative charge and hydrogen acquires partial positive charge in the two O−H bonds. Whereas in O−O bond, the electronegative is the same and thus there is equal attraction of a shared pair of electrons on both sides.
Hence, O−H bond is polar. But O−O bond is nonpolar
(D) CH4(methane)
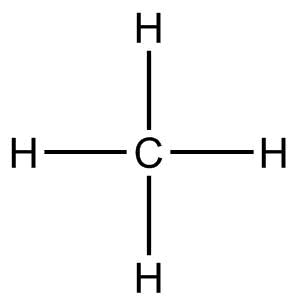
In the CH4molecule, the electronegativity difference between C and H is not much. Also, hydrogens are evenly distributed around carbon, so all the poles cancel out each other. Thus, CH4is a nonpolar molecule.
Hence, only H2O2 molecules have both polar and nonpolar bonds and hence the correct option is C.
Note: No compound is completely covalent or ionic. In covalent compounds, some ionic character exists. When a covalent bond is formed between homo atoms (similar atoms) like Cl2, O2 etc. then equal sharing of electrons occurs and a nonpolar bond is formed. But, in hetero atoms (different atoms) or hetero nuclear molecules like HCl, HF, etc. then the sharing electrons is unequal and polar bonds are formed.
