Question
Question: Which of the following contain \(P - P\) bond? (A) \[{P_2}O_7^{4 - }\] (B) \[{P_2}O_6^{3 - }\] ...
Which of the following contain P−P bond?
(A) P2O74−
(B) P2O63−
(C) P2O93−
(D) P2O64−
Solution
P-P bond means two phosphorus atoms are joined with each other and form a bridge between the molecules. P-P bond is a covalent bond, formed by the sharing of electrons by different atoms present in a molecule.
Complete step by step answer:
The prefix 'hypo-' means beneath or less than. Hypophosphoric acid has an oxidation state as +4 that is in between phosphoric acid and phosphorus acid. Thus, "hypophosphoric" refers to less than phosphoric but above the phosphorous acid.
Among the given options, hypophosphoric acid i.e. H4P2O64− is a mineral acid. It has a +4 oxidation state. The two phosphorus atoms of the molecule form a covalent P-P bond as shown in the figure.
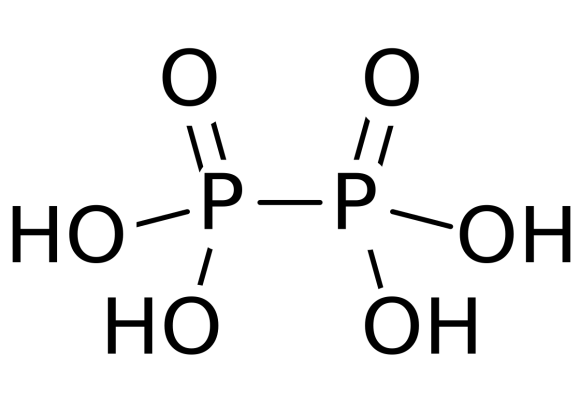
The structure of hypophosphorous acid is containing two P-O bonds with 151 pm length, P−OH bond with 151 pm length and P-P bond of 219 pm length. Hypophosphoric acid also has oxonium ions with formula [H3O+]2[H2P2O6]2− . The hypophosphoric acid is isostructural with the diammonium salt which containing [HOPO2PO2OH]2− anion with a P−P bond length of 219 pm.
Dihydrate H4O2P6.2H2O is the solid state of hypophosphoric acid, formed by the reaction of red phosphorus and sodium chlorite at room temperature.
2P+2NaClO2+2H2O→Na2H2P2O6+2HCl
Hence, the correct option is (A) P2O74−.
Additional information:
Hypophosphoric acid is used as a bleaching agent, reducing agent, stimulant in pharmaceutical agents and as a wetting agent. Hypophosphoric acid is triprotic and have dissociation constants pKa1= 2.2, pKa2= 2.8, pKa3= 7.3 and pKa4= 10.0.
Note:
In air the hypophosphites oxidise and form pyrophosphates containing the P2O74− ion where P has an oxidation state of +5. They are stable to alkali hydroxides. It converts to the orthophosphate when fused with sodium hydroxide.
