Question
Question: Which of the following configurations show the second excitation state of iodine? A. 
B. 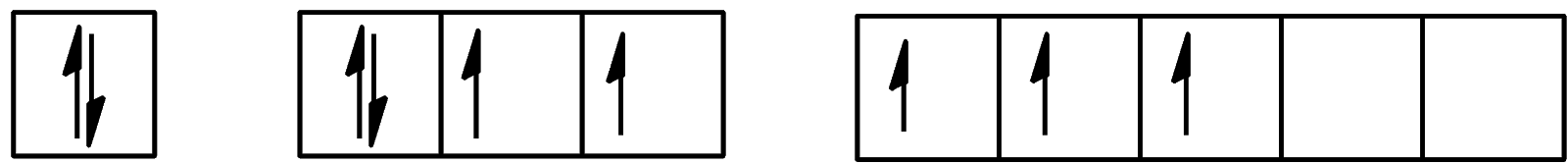
C. 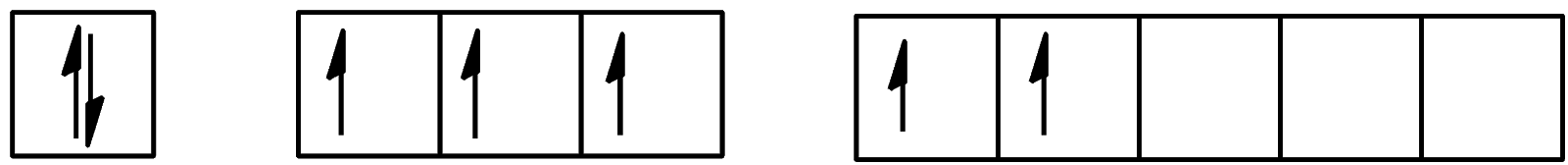
D. 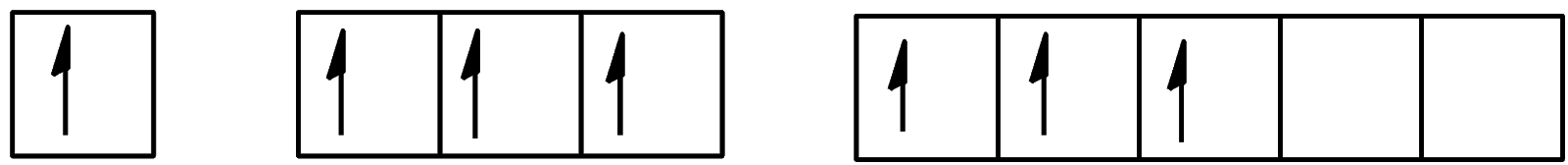
Solution
Think about the atomic number of iodine and the number of electrons that are present in its outermost shell. Determine the second excitation state according to this.
Complete step by step solution:
We know that the atomic number of iodine is 53, and its electronic configuration is denoted by [Kr]4d105s25p5. We will only consider the outermost valence orbitals of the atom, they are 5s25p5.
The atom is considered to enter its first excitation state when one electron from the outermost shell occupies a previously unoccupied orbital with the lowest energy. Here, the unoccupied orbital belongs to the 5d orbital. So, the electron from 5p will move to the 5d orbital. The same will happen in the second excitation state. An electron from 5p will move to the 5d orbital during this process too. Now, let us look at the ground state, the first excitation state, and the second excitation state.
- For the ground state, the electronic configuration will be:
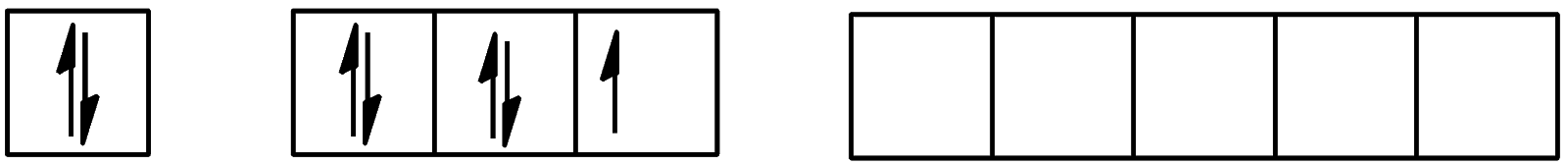
We see that no electrons are present in the d-orbital.
- For the first excitation state, the configuration will be:
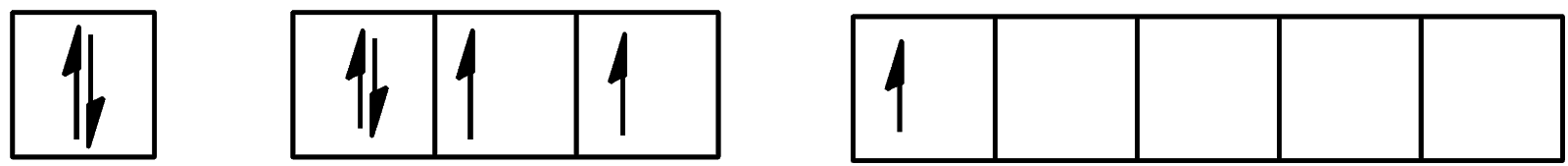
We can see that one electron is present in the d-orbital and 4 electrons are present in the p-orbital. The configuration will be [Kr]4d105s25p45d1.
- For the second excitation state, the configuration will be:
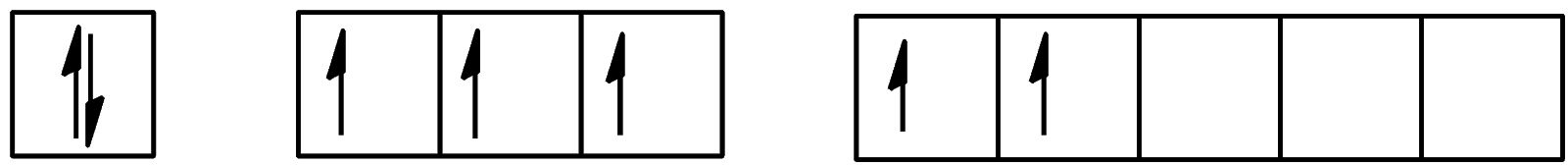
We can see that 2 electrons are present in the d-orbital and 3 electrons are present in the p-orbital. The configuration will be [Kr]4d105s25p35d2.
Hence, the correct answer to this question is option C.
Note: Remember that the electron that moves to the higher orbital will be the electron that has been written at the end while drawing the electronic configuration using Hund’s rule and Pauli’s exclusion principle. So the unpaired electron from the 5pz orbital will not move to the higher orbital but the paired electron from the 5py will move first and then the paired electron from the 5px orbital.
