Question
Question: Which of the following compounds will show the maximum enol content? (A)- \({\text{C}}{{\text{H}}_...
Which of the following compounds will show the maximum enol content?
(A)- CH3COCH2COCH3
(B)- CH3COCH3
(C)- CH3COCH2CONH2
(D)- CH3COCH2COOC2H5
Solution
Maximum enol content is shown by that compound in which enol form is stabilized by the hydrogen bonding between the oxygen atom and electronegative atom present in the molecule.
Complete Solution :
Discuss of the enol content in the above given compounds is as follow:
-In option (A) CH3COCH2COCH3 compound is given and in this compound keto functional group is converted into enol form by following manner:
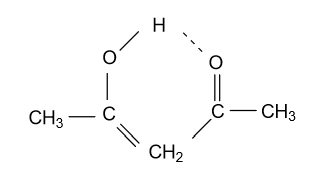
Here one carbonyl group or keto group converted into enol functional group and double bond is formed between carbonyl carbon and CH2 carbon. And this enol form is stabilized by the intramolecular hydrogen bonding between two carbonyl groups.
-In option (B) CH3COCH3 compound is given and in this compound keto functional group is converted into enol form by following manner:
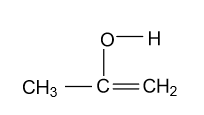
This enol form is not that much stable because near carbonyl group + I effect showing alkyl groups are present which decreases positive charge of carbonyl carbon.
-In option (C) CH3COCH2CONH2compound is given and in this compound keto functional group is converted into enol form by following manner:
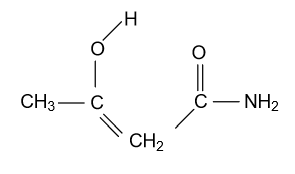
Here this enol form is also not that much of stable because with the second carbonyl group electron donating group NH2 is present, which decreases positive charge of carbonyl group carbon and there is not appreciable negative charge on carbonyl group oxygen so that they are not able to make intramolecular hydrogen bonding; due to which this compound doesn’t attain stability.
-In option (D) CH3COCH2COOC2H5compound is given and in this compound keto functional group is converted into enol form by following manner: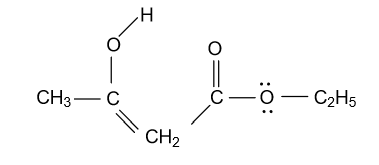
Here this enol form is also not that much stable because in this compound the second carbonyl group is present in resonance with the lone pair of electrons present in the near oxygen atom, that’s why the second carbonyl group is not involved in the formation of enol form.
Among all given compounds only CH3COCH2COCH3 shows high enol content because all two carbonyl group is involved in the formation of enol compound, through hydrogen bonding.
So, the correct answer is “Option A”.
Note: Here some of you may thought that why CH3COCH2CONH2 & CH3COCH2COOC2H5were not show high enol content while they also had two carbonyl groups. So the reason is that the second carbonyl group present in these compounds is not involved in the formation of stable enol form.
