Question
Question: Which of the following compounds will not undergo Friedel Crafts reaction with benzene? 
Solution
We know that Friedel Crafts reaction usually shows the addition of the halogen group to the alkyl ring. It usually gives two types of reaction one is alkylation, and the second is acylation reaction. They both are preceded by the electrophilic aromatic substitution.
Complete step-by-step answer : It is known to us that the electrophilic substitution reactions are carried out in the presence of catalysts that help in the generation of the electrophile. The reaction of benzene with haloalkanes in the presence of a catalyst, such as AlCl3 to give alkyl benzene are regarded as Friedel-Craft alkylation.
From all four given compounds, the option (C) compound generally will not undergo Friedel Crafts reaction with benzene because the compound (C) forms an intermediate carbocation. The carbocation will be dislocated, and the positive charge will be getting more electronegative at the sp2 hybridization carbon atom.
The carbonation form by compound C is shown below.
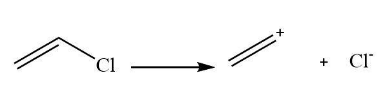
Note: The Friedel craft's reaction is one of the best reactions used for converting the alkyl ring to the halogen attached ring. It is very useful for the synthesis of several chemical compounds. It is also used for the manufacturing of organic compounds in the laboratory. The halogens are chlorine that is Cl, bromine that is Br, and fluorine that is F are usually used in Friedel craft’s reaction.
