Question
Question: Which of the following compounds will not be soluble in sodium bicarbonate? A) \( {\text{2,4,6 - T...
Which of the following compounds will not be soluble in sodium bicarbonate?
A) 2,4,6 - Trinitrophenol
B) Benzoic acid
C) o-Nitrophenol
D) Benzene sulphonic acid
Solution
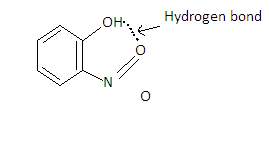
o-Nitro phenol has intramolecular hydrogen bonding which makes it difficult to release the H+ ion. So it is a weak acid which will not show any reaction when reacted with sodium bicarbonate. Hence it is not soluble in sodium bicarbonate.
Complete answer:
The given compounds will be soluble in sodium bicarbonate only if they have higher acidity. This means that they can easily give H+ ion and form sodium salts of acid. Here, 2,4,6 - Trinitrophenol has higher acidity than o-Nitrophenol as it has 3 - NO2 groups which is electron withdrawing group so it increases the acidic strength of phenol. So, it will be soluble in sodium bicarbonate. The reaction will be-
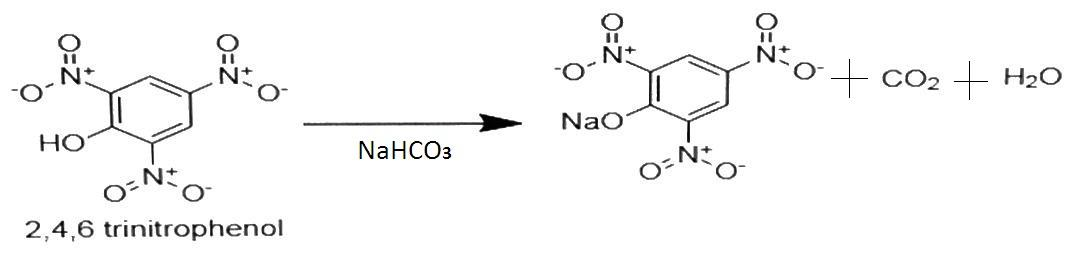
The product formed is the sodium salt of acid. Benzoic acid and Benzene sulphonic acid are strong acids as they give H+ ion easily. So they are soluble in sodium bicarbonate. The reaction is as follows-
\Rightarrow {{\text{C}}_6}{{\text{H}}_5}{\text{COOH + NaHC}}{{\text{O}}_3} \to {\text{Na}}{{\text{C}}_6}{{\text{H}}_5}{\text{COO + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\\
{{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{OH + NaHC}}{{\text{O}}_3} \to {{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{ONa + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\\
Hence the answer is ‘C’.
Note:
Hydrogen Bonding is the bonding between the hydrogen atom of one molecule with the electronegative atom of an adjacent molecule. It is represented by (-). Hydrogen bonding is of two types-
(i)Intermolecular hydrogen bonding- Hydrogen bond formed between 2 molecules
(ii)Intermolecular hydrogen bonding-Hydrogen bond formed within the molecule.
In o-Nitrophenol, Intermolecular hydrogen bonding is present.