Question
Question: Which of the following compounds will be suitable for Kjeldahl's method for nitrogen estimation. ...
Which of the following compounds will be suitable for Kjeldahl's method for nitrogen estimation.
| A) | 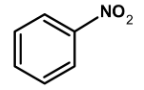 |
|---|---|
| B) | 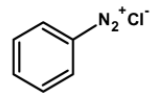 |
| C) | 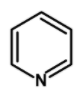 |
| D) | 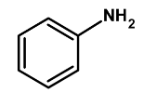 |
Solution
Kjeldahl’s method is one of the most commonly used methods for the estimation of nitrogen. It has a wide industrial application to estimate the nitrogen in the fertilizers, foodstuffs, drugs, etc. The method has certain limitations. It cannot be used for nitrogen-containing ring compounds .for example pyridine, quinoline, etc. and those which contain the nitrogen atoms directly bonded to the oxygen atom such as NO or azo compounds.
Complete step by step solution:
Kjeldahl's method is used for the estimation of the nitrogen atom. The Kjeldahl’s method is explained as follows,
Principle: A known mass of an organic compound is heated with conc. H2SO4 In presence of K2SO4 and a small amount of CuSO4 or mercury in Kjeldahl’s flask. The potassium sulphate increases the boiling point sulphuric acid and mercury or the copper sulphate catalyses the reaction.
On heating, the nitrogen present in the organic compound is converted into ammonium sulphate. The reaction of the nitrogen in the Kjeldahl’s method is as shown below,
N ( form organic compound) + conc.H2SO4 → (NH4)2SO4
The ammonium sulphate reacts with sodium hydroxide,
(NH4)2SO4 + 2 NaOH→ Na2SO4 + 2H2O + 2NH3
The ammonia gas liberated then again converted into the ammonium sulphate.
2NH3 +H2SO4→(NH4)2SO4
The volume of the acid which is not used up in the reaction is determined by the titration against the standard base solution. From this, the volume of ammonia used up is the volume of ammonia evolved which is equal to the amount of nitrogen present in the compound.
The method can be readily applied to the compound in which nitrogen is free to react with the reagent. The compounds which have nitrogen in the ring (like quinoline, pyridine, etc.), an azo compound, or in nitro compounds are not readily converted into the ammonium sulphate by the action of sulphuric acid.
Thus, the given compound aniline is suitable for Kjeldahl's method.
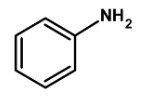
Hence, (C) is the correct option.
Note: Kjeldahl’s method has been widely used for the determination of nitrogen in the protein. However, it does not give the actual content of the nitrogen in the protein, as in food all nitrogen is not present in the form of protein. The proteins have different amino sequences; some might easily undergo the reaction but other amino sequences in which nitrogen is in the ring cannot be easily detected. The technique is time-consuming.
There is one more method for the determination of nitrogen. It is a dumas method.in this method, the organic compound containing nitrogen is heated in presence of copper oxide and liberates carbon dioxide and nitrogen gas. It is faster than Kjeldahl's method.
