Question
Question: Which of the following compounds show haloform reaction and racemization in \( O{D^ - }|{D_2}O \) . ...
Which of the following compounds show haloform reaction and racemization in OD−∣D2O .
A. CH3CH2OH
B.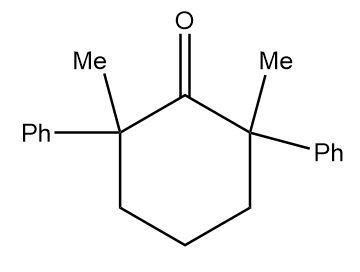
C.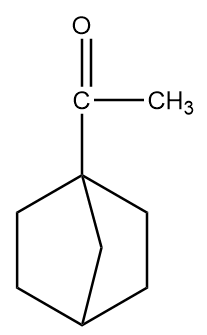
D.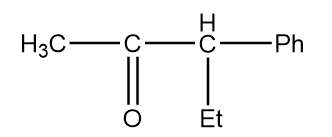
Solution
Hint : Haloform reaction: It is the reaction in which methyl ketone reacts with dihalogen in the presence of dilute sodium hydroxide solution and formation of haloform i.e., CHX3 takes place along with the removal of sodium salt of acid.
Complete Step By Step Answer:
The major factor for a compound to undergo haloform reaction is that a methyl group must be attached to the carbonyl group. Let’s test the haloform reaction for each given option.
Structure given in option (A)- CH3CH2OH :
Ethyl alcohol when reacts with dihalogen in the presence of base, then it first gets oxidized under such conditions and forms ethanal which on halogenation reaction gives a yellow precipitate. Hence, ethyl alcohol gives a positive test for halogenation reaction. The reaction proceeds as follows:
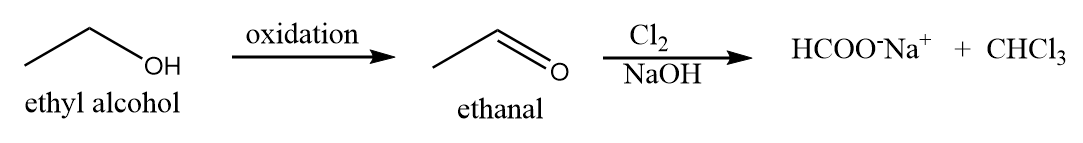
Structure given in option (B)-
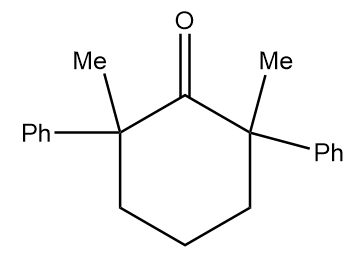
As in the given compound, no methyl group is present at the adjacent position to the carbonyl group, so it will not show a positive haloform test.
Structure given in option (C)-
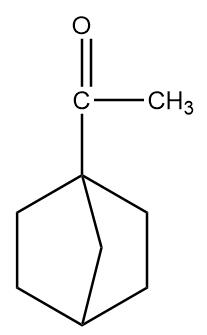
As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Structure given in option (D)-
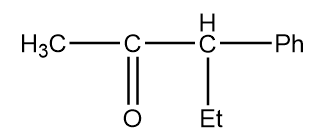
As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Now, for the racemization reaction, when the acidic hydrogen is extracted by OD− then the carbon atom becomes sp2 hybridized i.e., the compound becomes planar and resonance stabilized. Therefore, the attack of electrophile is possible from the front as well as back side of the carbon atom and hence racemization of products will take place. This case is only possible in structure (D). The reaction is as follows:
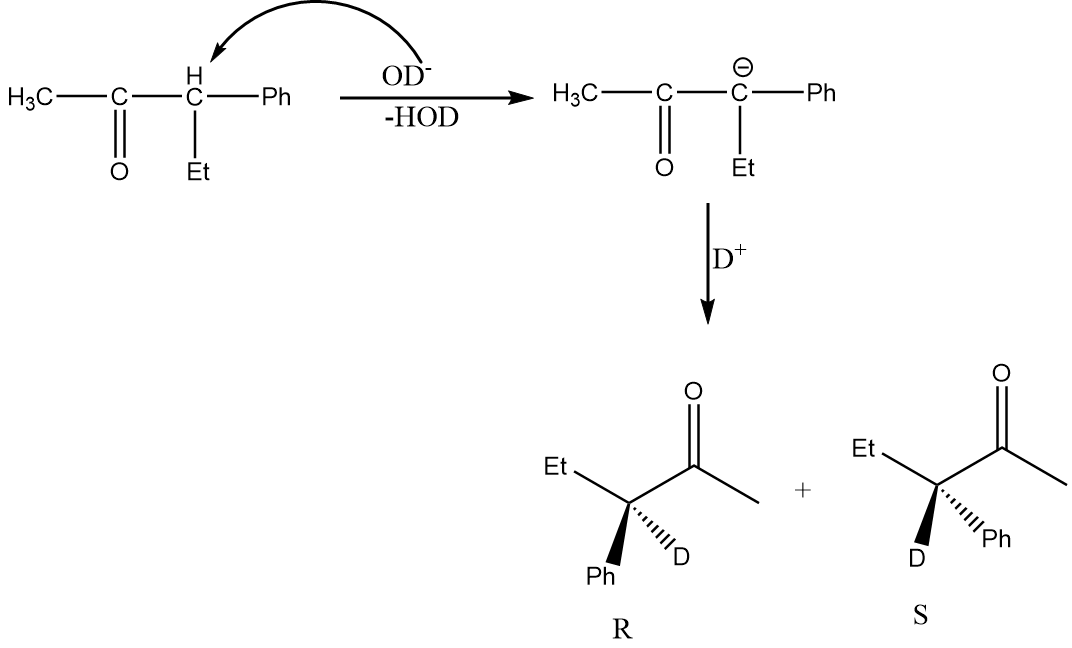
Hence, option (D) is the correct answer.
Note :
It is important to note that the Racemization process can be done in the molecule which consists of at least one chiral carbon. Therefore, although methyl groups consist of more acidic hydrogen, the racemization takes place at the carbon atom which is connected to four different groups and formation of 50% R form and 50% S form is observed which is known as a racemic mixture.
