Question
Question: Which of the following compounds show dipole moment? This question has multiple correct options. ...
Which of the following compounds show dipole moment?
This question has multiple correct options.
(A)1,4−dichlorobenzene
(B)1,2−dichlorobenzene
(C)1,3−dichlorobenzene
(D)trans−2,3−dichloro−2−butene
Solution
When there is a separation of charge, the dipole moment is noticed. It can be noticed between ions in an ionic bond or between atoms in a covalent bond. Dipole moments arise from the differences in the electronegativity of the atoms. The dipole moment is simply the measure of the polarity of a molecule.
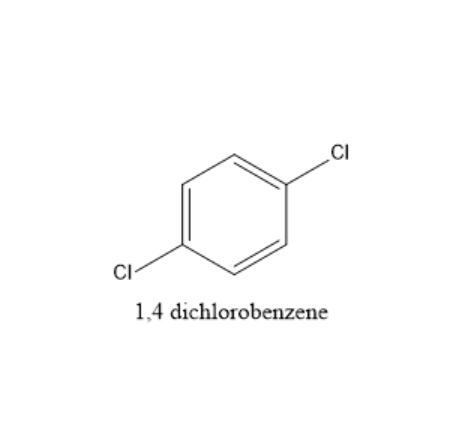
Complete answer: In 1,4−dichlorobenzene, all carbons are sp2 hybridized. It means all carbons have a double bond in which one bond is a sigma bond and one bond is a pi bond. As the hybridization is sp2 it is planar. The two chlorine atoms are in para position. The electronegativity of the carbon hydrogen bond is less and is considered as a non- polar covalent bond. The dipole moment of two carbon and chlorine bonds are in equal and opposite directions. So, the total dipole moment will be zero. The structure of 1,4−dichlorobenzene is given below:
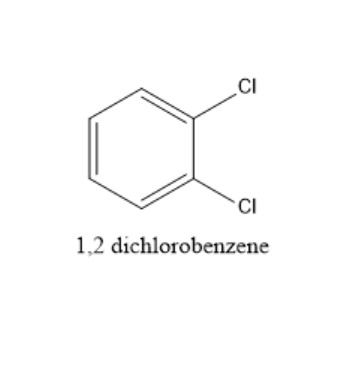
In 1,2−dichlorobenzene, dipole moment is present because the two carbon chlorine bonds are at 60∘ to each other. The structure of 1,2−dichlorobenzene is given below:
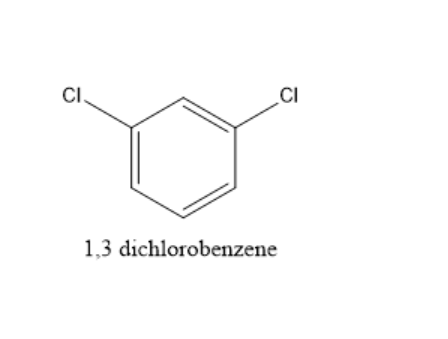
In 1,3−dichlorobenzene, dipole moment is present and the structure of 1,3−dichlorobenzene is given below:
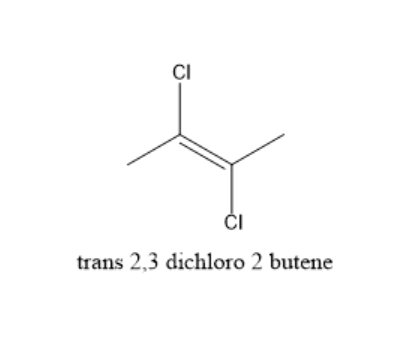
In trans−2,3−dichloro−2−butene, the two chlorine atoms are present on opposite sides. The hybridization is sp2.So, it is planar. The total dipole moment is zero as the dipole moment due to two chlorine atoms is equal and opposite in direction.
Therefore, option B and C are the correct options.
Note:
The dipole moment between single bonds of a diatomic molecule is known as bond dipole moment. The total dipole moment in a polyatomic molecule is the vector sum of the bond dipoles. The dipole moment is zero if the two opposite bond dipoles cancel each other.
