Question
Question: Which of the following compounds react with \[{\text{NaN}}{{\text{O}}_{\text{2}}}\] and \[{\text{HCl...
Which of the following compounds react with NaNO2 and HCl at 0 - 4∘C to give alcohol/phenol?
A. C6H5NH2
B. C2H5NH2
C. CH3NHCH3
D. C6H5NHCH3
Solution
The reagent NaNO2 and HCl used to distinguish primary, secondary and tertiary amines. Only primary aliphatic amines react with NaNO2 and HCl at 0 - 4∘C . The product of the reaction is alcohol.
Complete Step by step answer: The reagent given to us is NaNO2 and HCl and the reaction condition is 0 - 4∘C. Only primary aliphatic amines react with NaNO2 and HCl at 0 - 4∘C and give alcohol as the product.
The amine given in option A is C6H5NH2. Its structure is as follows:
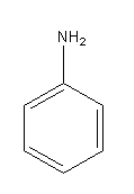
It is aromatic amine so it will not give phenol after reacting with NaNO2 in HCl at 0 - 4∘C.
Thus, option (A) C6H5NH2 is incorrect.
The amine given in option B isC2H5NH2. Its structure is as follows:
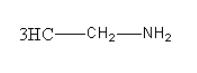
It is a primary aliphatic amine so it will give alcohol after reacting with NaNO2 in HCl at 0 - 4∘C.
So, option (B) C2H5NH2is correct.
The amine given in option C is CH3NHCH3. Its structure is as follows:
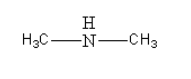
It is secondary aliphatic amine so it will not give alcohol after reacting with NaNO2 in HCl at 0 - 4∘C.
Thus, option (C) CH3NHCH3 is incorrect.
The amine given in option D is C6H5NHCH3. Its structure is as follows:
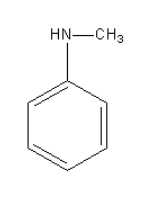
It is aromatic secondary amine so it will not give phenol after reacting with NaNO2 in HCl at 0 - 4∘C.
Thus, option (D) C6H5NHCH3 is incorrect.
Hence, option (B) C2H5NH2is the correct answer.
Note: Aliphatic primary amines react withNaNO2 in HCl at 0 - 4∘Cand undergo diazotization to form alkane diazonium salt, which however being unstable decomposes to form a mixture of alcohols, alkene with the liberation of N2 gas. Secondary amines react with NaNO2 in HCl to form N-nitrosamines.
