Question
Question: Which of the following compound’s prefix ‘iso’ is not correct- (A) Isopentane (B) Iso hexane (...
Which of the following compound’s prefix ‘iso’ is not correct-
(A) Isopentane
(B) Iso hexane
(C) Isobutane
(D) Iso octane
Solution
Hint : All the options which are given are alkanes. Alkanes are the hydrocarbons which have only a single bond. According to IUPAC nomenclature, they are named according to the number of carbon atoms present in the compound. The prefix ‘iso’ is used for naming branched alkyl chains. In other words, a methyl group is attached as a branch on the second carbon of a carbon chain. The prefix ‘iso’ will be placed in front of the alkane indicating the total number of carbons.
Complete Step By Step Answer:
The naming of the organic compound is done according to the nomenclature rules and standard given by IUPAC. So, according to IUPAC, the longest possible carbon chain is called the parent chain. The chains which are not included in the parent carbon chain are called a side or branched chain. Numbering should be done so that carbon having branches will get the lowest possible number. Alkanes are named according to the number of carbon atoms present. Example – methane (1 carbon), ethane (2 carbon) etc.
The ‘iso’ prefix is used for naming the alkyl chains i.e. the branch of alkyl group on the carbon atom. This ‘iso’ prefix is considered as a fundamental name and it is written alphabetically. This ‘iso’ prefix is used only for the compound which has 4 , 5 and 6 carbon atoms only. After this, we do not use the prefix ‘iso’.
In the given question:
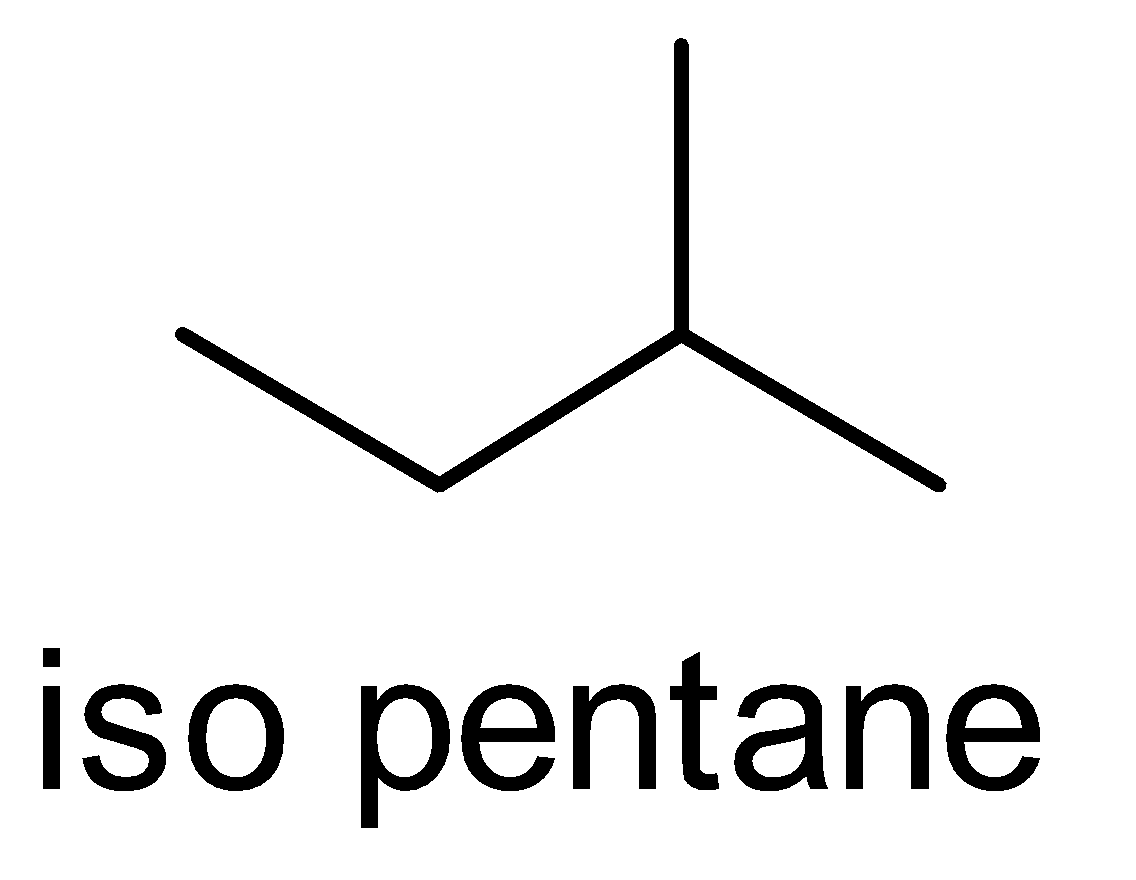
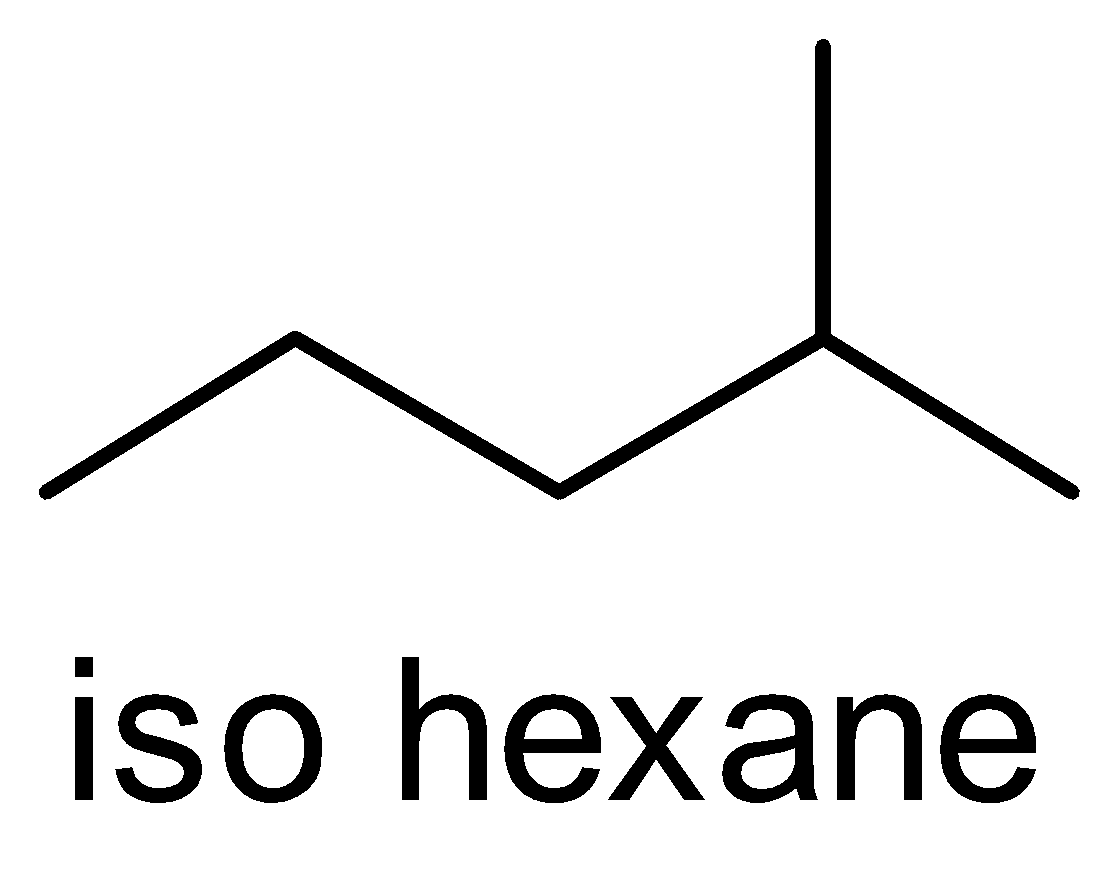
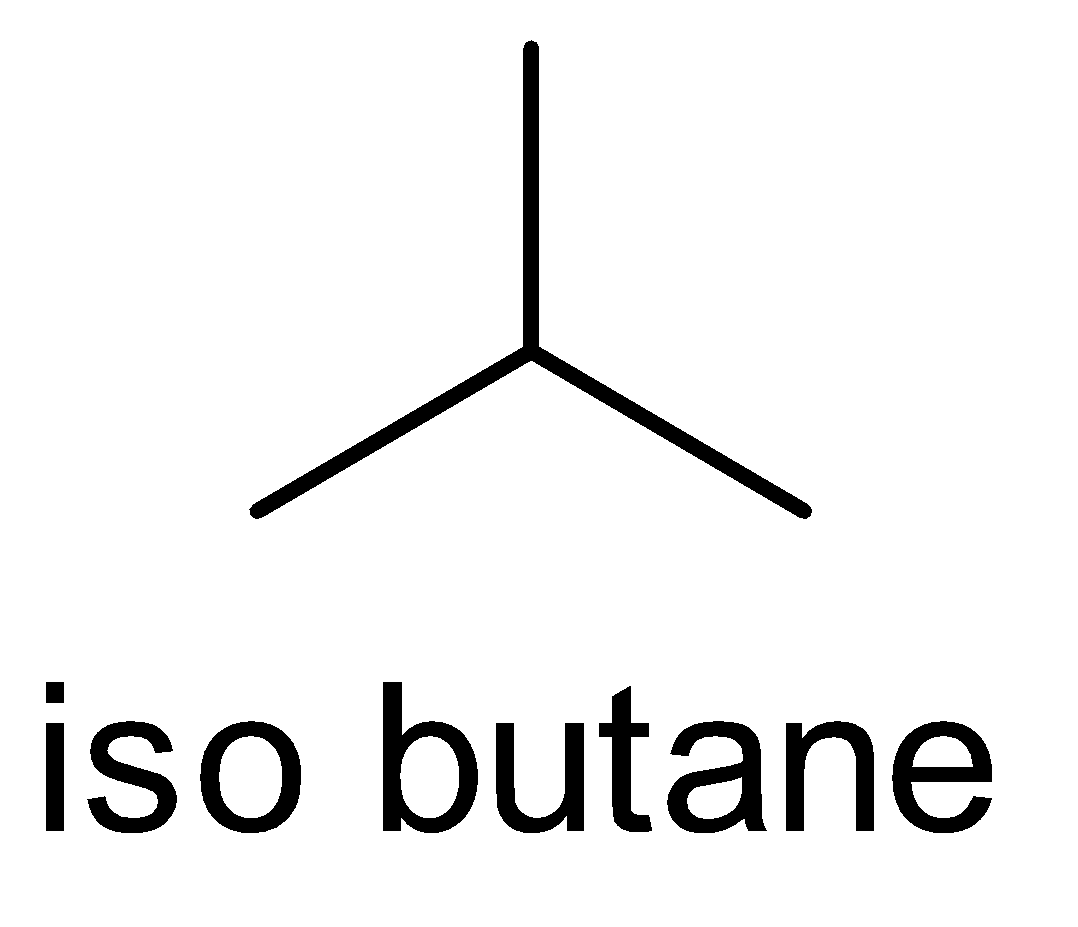
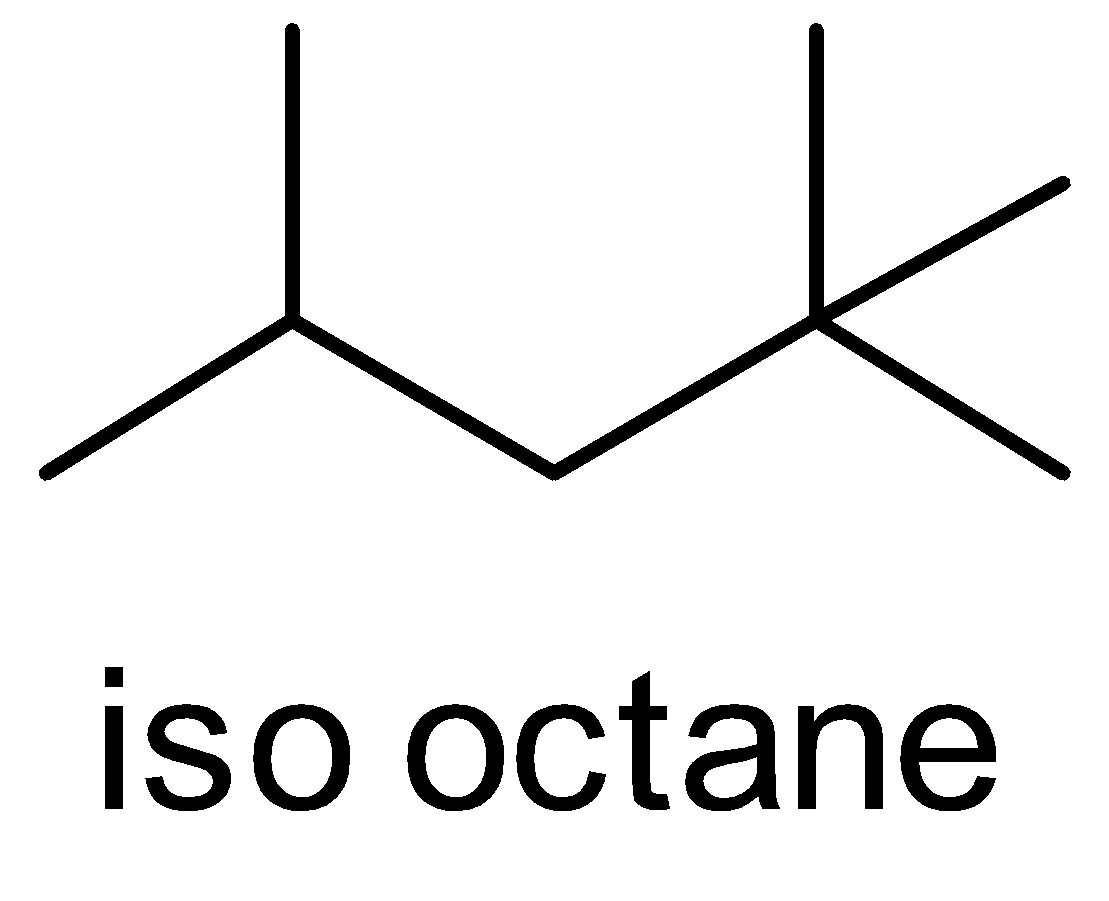
So, according to the IUPAC nomenclature, the ‘iso’ prefix is not used for iso octane.
Hence, the correct answer is option (D).
Note :
The prefix ‘iso’ is used only for 1 methyl branch on the carbon atom, while the prefix ‘neo’ is used for methyl branches in consecutive adjacent carbon atoms. When the alkyl branches have a functional group, then we use the prefix ‘sec’ and ‘tert’.
