Question
Question: Which of the following compounds might be useful to the chemist trying to increase the optical purit...
Which of the following compounds might be useful to the chemist trying to increase the optical purity of (d) sample?
(a)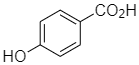
(b)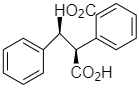
(c)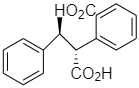
(d)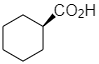
Solution
Substances which rotate the plane of polarized light are optically active substances. The isomer which rotates the plane of polarized light to the right is called dextro rotatory designated as ( d ) and the one which rotates the plane of polarized light to the left is called laevo rotatory designated as (l).
Complete answer:
Optical purity is a measure of its enantiomeric purity. Optical purity explains how much of one enantiomer is present in excess in the racemic mixture. Enantiomeric molecules rotate the plane in opposite directions but with the same magnitude. Optically active isomers are mirror image compounds and non-superimposable on each other and do not possess the plane of symmetry.
These optical isomers also have the property of Chirality. The necessary condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure.
Optically active compounds are used for resolution of ± mixture.
Option (b) is optically active
And the other options present are optically inactive.
meso compound compounds have chiral centres but they do not show optical activity due to internal compensation, It is superimposed on its mirror image.
Therefore the correct answer is option (b).
Note:
A 1:1 equilibrium mixture of d and l isomers gives a net zero rotation and is called a racemic mixture. A racemate is optically inactive as there is no net rotation of plane-polarised light.
However the enantiomers rotate plane-polarised light in opposite directions, the rotations cancel out because they are present in equal amounts.
