Question
Question: Which of the following compounds is optically active? A. \(\text{C}{{\text{H}}_{3}}\text{C}{{\text...
Which of the following compounds is optically active?
A. CH3CH2COOH
B. HOOC - CH2- COOH
C. CH3CH(OH)COOH
D. Cl2CHCOOH
Solution
A substance which has optical activity, is a substance which rotates the plane of plane polarized light. To know whether a compound is optical active we need to consider the property of chirality. By chirality we mean a carbon having 4 different groups attached to it. If we find any chiral centre, then that compound is optically active. With this approach, we can find out which of the compounds mentioned in the options are optically active.
Complete step by step answer:
Option A mentions the compound CH3CH2COOH which has an IUPAC name of propanoic acid has a molecular structure of,
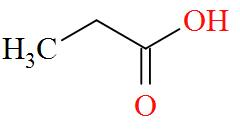
As per the definition, no carbon atom in propanoic acid is seen to be bonded to four different atoms or groups of atoms. Hence, propanoic acid is optically inactive in nature.
Option B mentions the compound HOOC - CH2- COOH also known as Malonic acid has a molecular structure of,
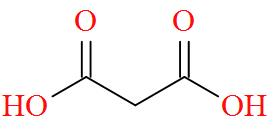
As per the definition, no carbon atom in Malonic acid is seen to be bonded to four different atoms or groups of atoms. Hence, Malonic acid is optically inactive in nature.
Option C mentions the compound CH3CH(OH)COOH which has an IUPAC name of 2-hydroxypropanoic acid, also known as lactic acid has a molecular structure of,
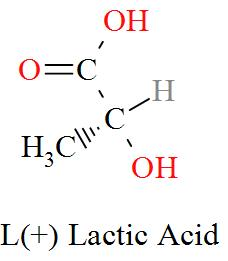
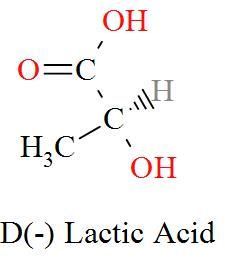
As per the definition, here we can see one carbon atom in Lactic acid is seen to be bonded to four different atoms or groups of atoms forming a Levo and a Dextro structure. Hence, Lactic acid is optically active in nature.
Option D mentions the compound Cl2CHCOOH which has an IUPAC name of dichloroacetic acid has a molecular structure of,
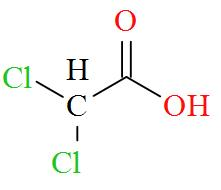
As per the definition, no carbon atom in dichloroacetic acid is seen to be bonded to four different atoms or groups of atoms. Hence, dichloroacetic acid is optically inactive in nature.
Therefore, the correct option is Option C.
Note: Therefore, we can say that chiral molecules are optically active, which implies that when a beam of plane polarized light passes through a chiral molecule. This will make sure that it interacts with the molecule in such a way that the angle of the plane of oscillation rotates.
