Question
Question: Which of the following compounds is not formed in the iodoform reaction of acetone? (A) \({ CH }_{...
Which of the following compounds is not formed in the iodoform reaction of acetone?
(A) CH3COCH2I
(B) ICH2COCH2
(C) CH3COCHI2
(D) CH3COCI3
Solution
Iodoform reaction of acetone will lead to the products - acetic acid (CH3COOH) (sodium acetate) and iodoform (CHI3). On writing the mechanism of the reaction, we can find certain stable intermediates are also formed. None of these intermediates contains a C=C bond. So, among the options given, the compound with C=C is the answer.
Complete Step by Step Solution:
-Structure of acetone is given below:
-Iodoform reaction is used to identify the presence of carbonyl compounds in compounds.
-Here in the iodoform reaction of acetone, the acetone will be reacted by I2 in the presence of NaOH to form acetic acid (sodium acetate) and iodoform.
-When we look at the mechanism of this reaction, we can see many intermediates formed in between.
-Given below is the diagrammatic representation of the mechanism of iodoform reaction of acetone.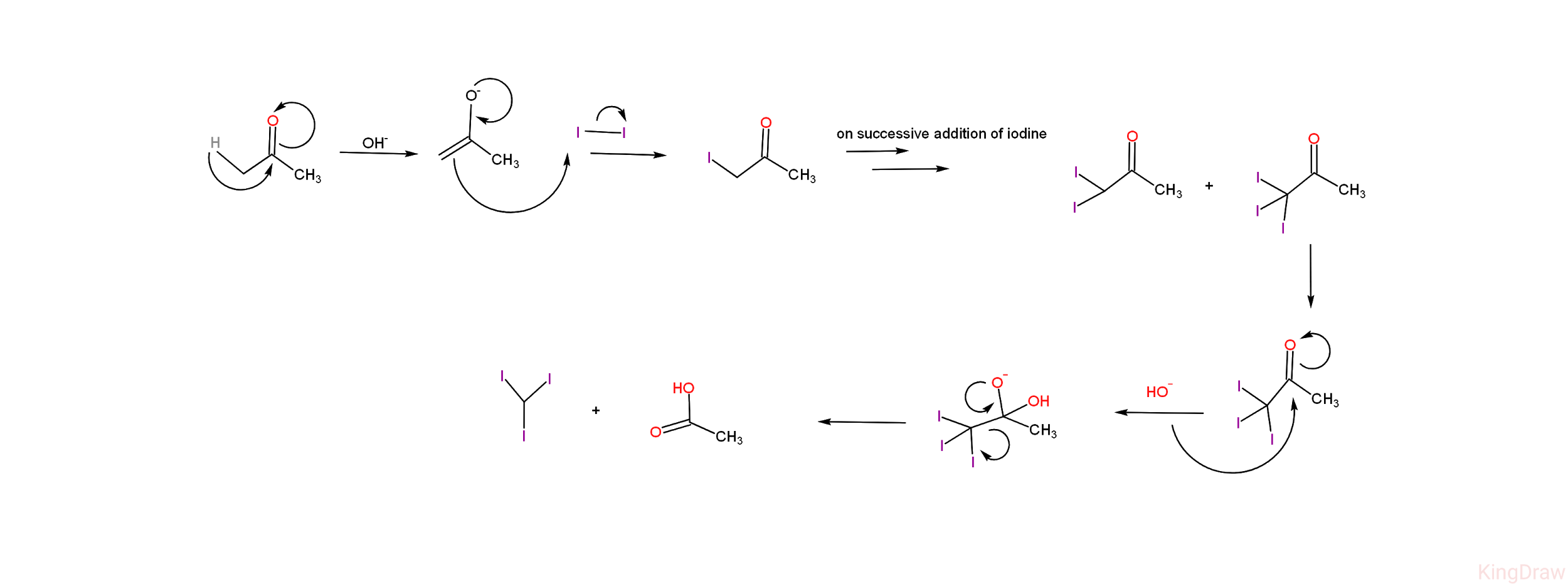 -From the mechanism, we can see that the iodoform reaction of acetone leads to the products - iodoform (CHI3) and acetic acid (CH3COOH ).
-From the mechanism, we can see that the iodoform reaction of acetone leads to the products - iodoform (CHI3) and acetic acid (CH3COOH ).
-Apart from the final products, there are certain stable intermediates formed in the reaction - i.e, on addition of iodine on acetate ion, it leads to the formation of 1-iodoacetic acid (CH3COCH2I) and on successive addition of iodine , two more compounds are formed which are 1,1-di iodoacetic acid (CH3COCHI2 ) and 1,1,1- tri iodoacetic acid (CH3COCI3)
-So, these compounds CH3COCH2I,CH3COCHI2 , CH3COCI3are formed in the iodoform reaction of acetone, which are satisfied by Option (A), option (C) and option (D).
-In the question, it has been asked which among the options are not formed in the iodoform reaction of acetone and that compound is Option (B)ICH2COCH2.
Therefore, the correct answer here is Option (B)ICH2COCH2.
Note: Iodoform reaction occurs when there is an α- hydrogen in that compound, and this α - hydrogen will be substituted with iodine leading to the formation of iodoform. In the case of acetone, there are three α - hydrogens present in the compound, so all the three α - hydrogens are replaced by iodine successively and thereby these stable intermediates are formed.
