Question
Question: Which of the following compounds is not chiral? (A) \( DC{H_2}C{H_2}C{H_2}Cl \) (B) \( C{H_3}C...
Which of the following compounds is not chiral?
(A) DCH2CH2CH2Cl
(B) CH3CHDCH2Cl
(C) CH3CHClCH2D
(D) CH3CH2CHDCl
Solution
Hint : If a carbon atom is bonded to four different substituents or groups, it loses all its symmetry. It is known as the asymmetric carbon centre. This configuration is known as Chirality and the molecule may exist as the Right-Handed Configuration or Left-Handed Configuration. This is called enantiomorphism, where the compounds are mirror images to each other and are non-superimposable on each other. Molecules are said to be chiral if they are mirror images that are non-superimposable to each other. Hence the molecules with non-identical mirror images are said to be chiral.
Complete Step By Step Answer:
To determine whether the molecule is chiral at least one carbon atom should be a stereogenic centre i.e., attached to four different substituents.
The first molecule given is DCH2CH2CH2Cl - In this molecule, every carbon is attached to two same Hydrogen atoms. Hence no carbon atom is attached to four different substituents. Hence none of the carbon atoms is Chiral.
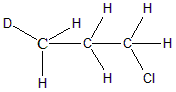
In the next molecule CH3CHDCH2Cl the second carbon atom is attached to one H, one D and two other different groups. Hence C2 carbon is said to be achiral. Option b is incorrect because chiral carbon is present.
CH3CHClCH2D also has a chiral carbon centre which is the second carbon itself. Hence it also cannot be achiral. Option (c) is incorrect.
In this molecule CH3CH2CHDCl the third carbon atom is attached to one H, one Cl, one D and another group. Which means that it is chiral. Option (d) is also incorrect.
The correct answer is Option (a).
Note :
The centre of stereoisomerism is the stereogenic centre which can be a point, axis or plane. Stereogenic elements may be chiral or achiral. Alkenes have an achiral stereogenic centre wince substitution of even one atom on either carbon can change the configuration from cis to trans.
