Question
Question: Which of the following compounds is most acidic? A: \(Cl - C{H_2} - C{H_2} - OH\) B: 
C: 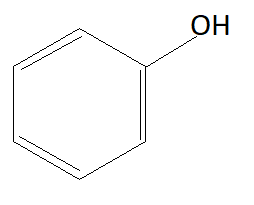
D: 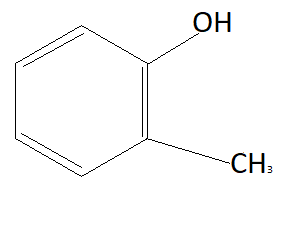
Solution
Alcohols are less acidic than phenols. Acidic character of a compound also depends upon the type of groups that are present in a compound. There are two types of groups: electron withdrawing group and electron donating group.
Complete step by step answer:
As we know phenols are more acidic than alcohols because of phenoxide ions. When phenols lose their hydrogen phenoxide ion is formed which is highly stable due to which phenols readily lose their hydrogen atom and form phenoxide ion. Chemical formula of phenoxide ion is C6H5O−. Among given options option A is alcohol and rest of the options are phenols. This means option A is not the answer.
Now the option will be eliminated on the basis of the electron withdrawing group. Alkyl group that is CH3 is an electron donating group. This means it will donate electrons to the benzene ring and reduce the chances to pull electrons from hydrogen. This means it is increasing the bond strength of O−H which reduces the possibility of losing hydrogen ions. This means option D is also not the answer (as it contains an electron donating group). NO2 is an electron withdrawing group that means it will pull electrons from the benzene ring and increases the possibility of taking electrons from hydrogen atoms. Therefore phenol having NO2 group is more acidic than phenol. So correct answer is option B that is:
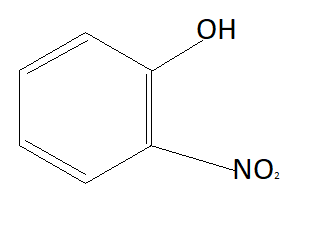
Note:
Phenoxide ion is highly stable due to resonance. Resonance refers to delocalization of charge. This means in phenoxide ion charge is not limited to one atom only it keeps on moving to different atoms which increases the stability of a molecule.
