Question
Question: Which of the following compounds is aromatic? A.
B.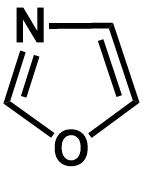
C.
D.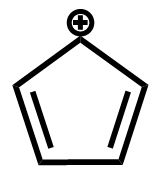
Solution
Aromaticity can be determined by calculating the number of π electrons being delocalized in the structure and then using Huckel’s rule of aromatic compounds. The Huckel’s rule states a set of rules or parameters that must be fulfilled by an organic compound in order to be called aromatic.
Complete step by step answer:
The Huckel’s rule of aromaticity states that:
A compound is aromatic only if it contains cyclic and continuous delocalization or conjugation of π electrons.
The compound must be planar which means that all the carbon atoms must lie in a single plane.
The organic compound must contain 4n+2 number of π electrons where the value of n can be any whole number.
If all these three conditions stated above are not fulfilled then the compound is not aromatic.
The first compound contains a total of four electrons which does not satisfy the 4n+2 condition and is therefore not an aromatic compound.
The second compound contains a total of six electrons being delocalized in a planar ring, which makes it an aromatic compound.
The third compound has two electrons but one of the three carbon is not part of the cyclic conjugation, therefore it’s not an aromatic compound.
The fourth compound contains a total of six electrons being delocalized in a planar ring, which makes it an aromatic compound.
⇒ Hence, the second and the fourth compounds are aromatic which means that option (b) and (d) are correct.
Note:
The second compound contains nitrogen as well as oxygen as the members of the ring. Though the oxygen atom contains two lone pairs, it only contributes a single lone pair for the conjugation and lone pair on nitrogen does not participate in conjugation at all as it is double bonded.
