Question
Question: Which of the following compounds is achiral? A.
B.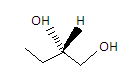
C.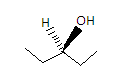
D.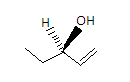
Solution
Chiral carbon is the carbon in which all four valencies of carbon are satisfied by different elements. If even one valency is satisfied by different elements then the carbon is said to be achiral. So first we will check if there is a chiral carbon present in the given compounds or not.
Complete step by step answer:
We have to find which compound is achiral.
The compounds that do not have a chiral carbon will be achiral molecules or compounds. So first we will check if there is a chiral carbon present in the given option one by one.
First, the compound in A is butan - 2 - ol (CH3−CH(OH)−CH2−CH3)whose structure is given as-
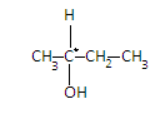
Here we can see in the structure that the second carbon is the chiral compound as its all four valencies are satisfied by different elements. It is the chiral center and is indicated by (*) sign. So it is not achiral.
The second compound is Pentane - 1,3 - diol. Its structure is given as-
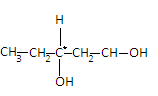
We can see in the structure that the carbon in the center has different elements attached to it. So it is a chiral compound.
The third compound is Pentane - 3 - ol and its structure is given as-
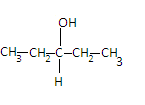
Here we can see that the two valencies of the center carbon are satisfied by two same ethyl groups so it is not a chiral carbon. Hence the compound is achiral.
The fourth compound is Penten - 3 - ol and its structure is given as-
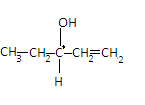
Here all the valencies of the carbon are satisfied by different elements so the carbon in the center is a chiral carbon. So this compound is not an achiral compound.
Hence the correct answer is C.
Note:
We can also find the achiral compound by this method-
-First, draw the mirror image of the compound and then see if the two molecules are the same or different.
- If the mirror image of the compound is the same as the image of the compound then it is chiral.
-If the mirror image of the compound is different, then it is achiral.
