Question
Question: Which of the following compounds have higher enolic content than ketone content? A)  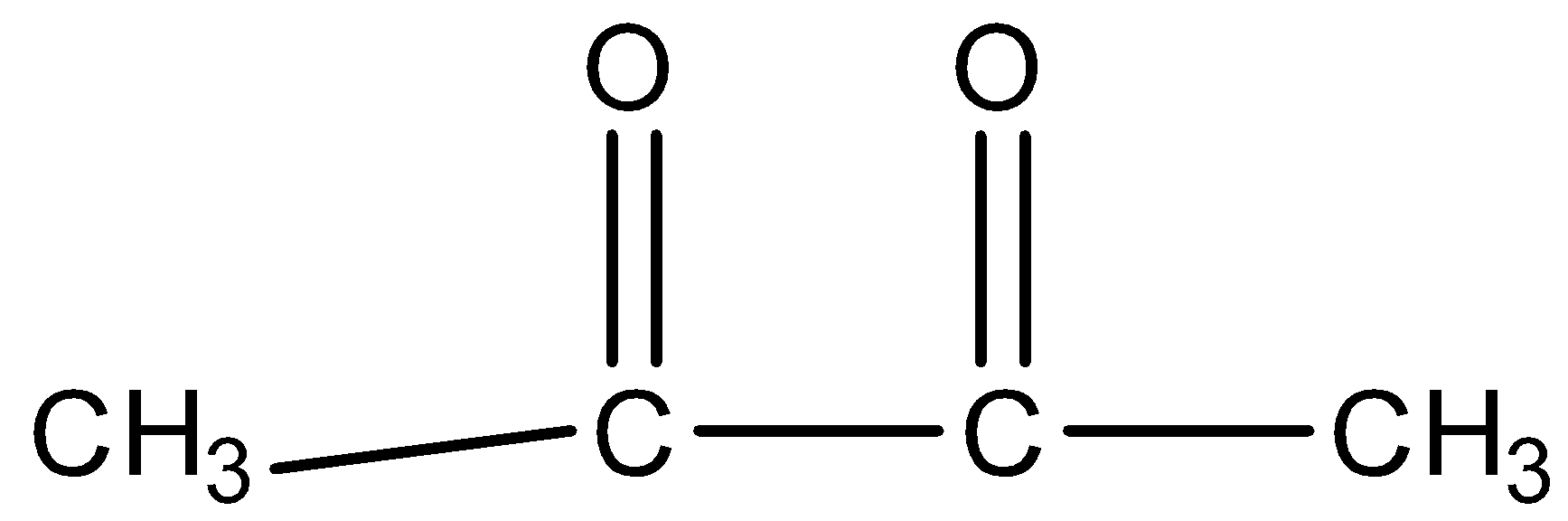
B) 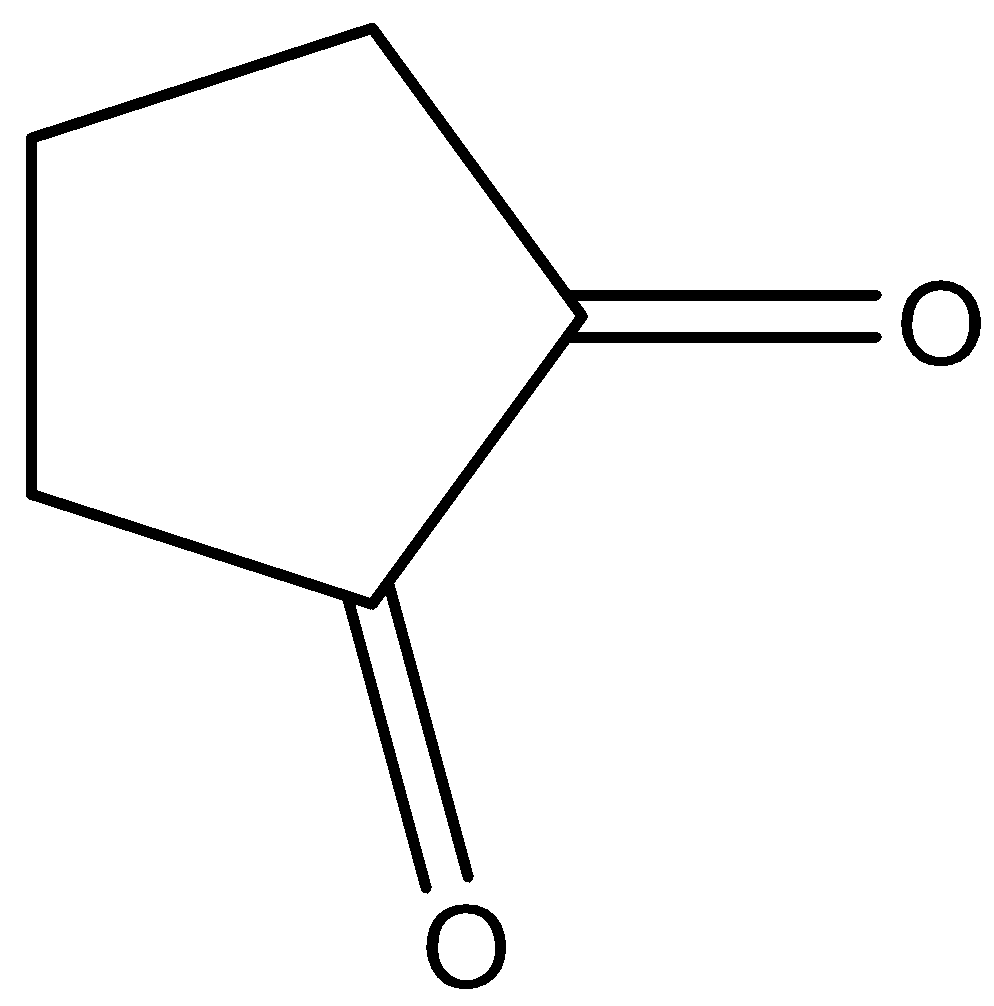
C) 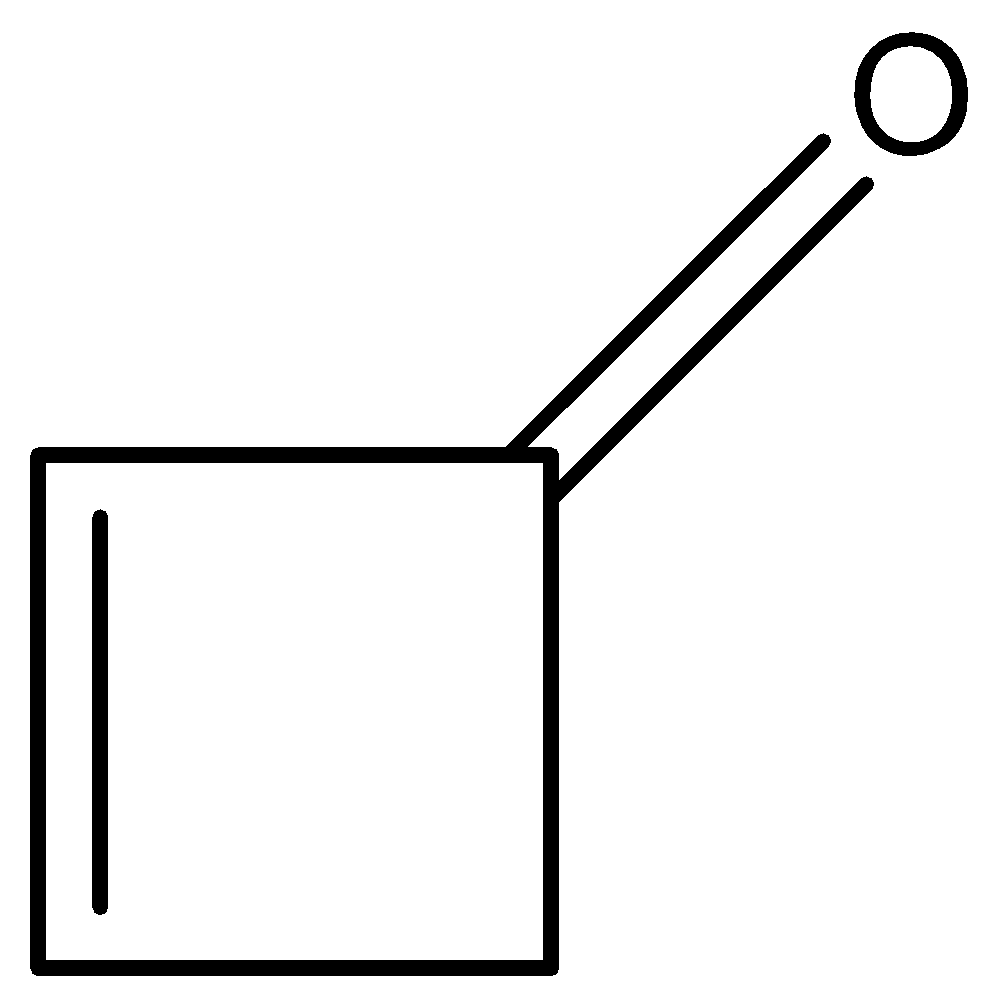
D) 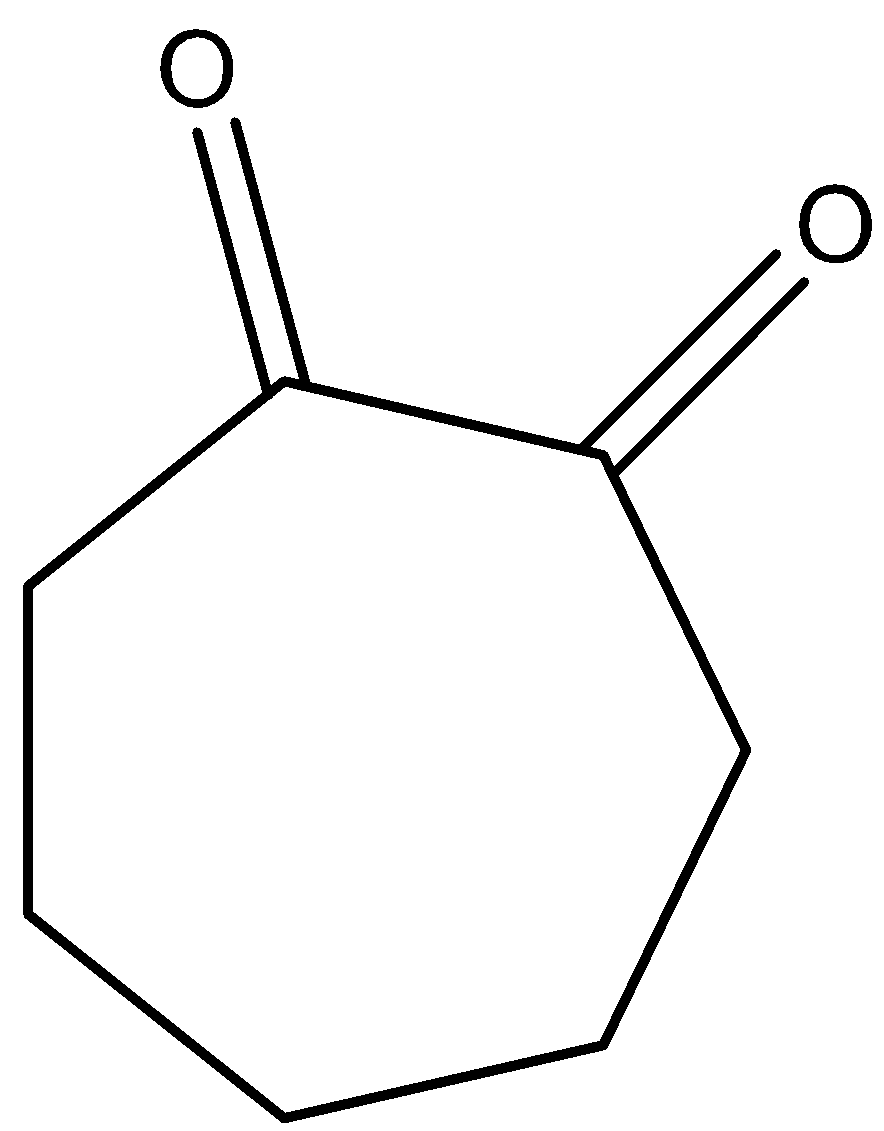
Solution
We must have to know that in this question we need to know what is enolic content and ketone content to solve this question. Enolic content can be identified on the basis of an ene and ol representing alcohol, which together forms an enol. Keto enol tautomerism is the process which has chemical equilibrium between ketone and enol form.
Complete answer:
Higher enolic content can be seen with the property of tautomerism in the following compounds. All given compounds have a ketonic group present in them, we can identify it with the help of C=O that represents a ketone functional group. The compound in which enolic content is higher can be looked at if it is easy for tautomerism. So we will check for the options given when we look at option B so on keto enol tautomerism we get this product.
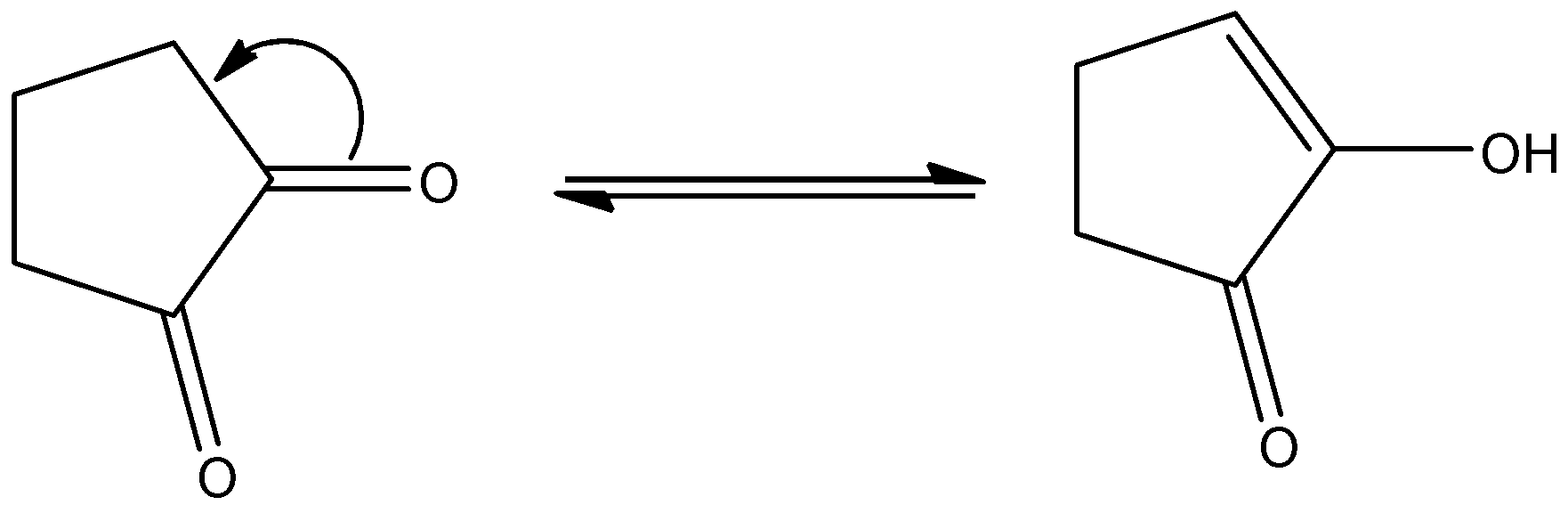
So on keto enol tautomerism we get a stable product only in this option while all other options hinder the tautomerism.
Thus option B is the correct option as shown in the reaction above, as due to the presence of hydroxyl group and oxygen there is a possibility of hydrogen bonding which is also there in the other other options but higher will be for this system.
Note:
We must have to know that the keto enol tautomerism is the process which has chemical equilibrium between ketone and enol form. And these keto and enol forms are known as tautomers in this case. Due to the presence of acidic hydrogens a molecule containing carbonyl group undergoes a proton-transfer equilibrium which is called tautomerism.
