Question
Question: Which of the following compounds has zero dipole moment? (A) \({ 1,4-dichlorobenzene }\) (B) \({...
Which of the following compounds has zero dipole moment?
(A) 1,4−dichlorobenzene
(B) 1,2−dichlorobenzene
(C) 1,3−dichlorobenzene
(D) 1−chloro−2−methylbenzene
Solution
The bond dipole moment uses the idea of an electric dipole moment to measure the polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges. The bond dipole μ is given by: μ=δd
Complete step by step solution:
As there are two Cl groups present in ortho makes an acute angle according to the equation;
Dipole moment, D = d12+d22+d1d2cosx
where, x = angle between the groups
A. 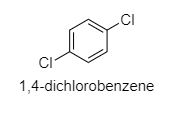
Here, the dipole moment is 0 as Cos90∘ = 0.
B. 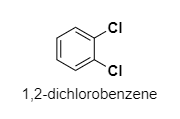
The dipole moment of 1,2−dichlorobenzene is 2.54D,so
C. 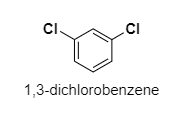
The dipole moment of 1,3−dichlorobenzene is 1.72D.
D. 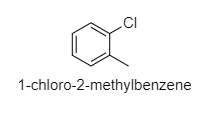
Here, there will be some dipole moment, not zero.
The order of dipole moment is: 1,2−dichlorobenzene 1,3−dichlorobenzene 1,4−dichlorobenzene.
Trans-dichlorobenzene has zero dipole moment as it is symmetrical and the dipole moment will cancel out.
Hence, the correct option is A.
Factors affecting dipole moment:
- Polarity of molecule
- Magnitude of charge
- Geometry of molecule
- The boiling point of the element.
- Position of bond and lone pair of electrons in a molecule.
- The dipole moment depends upon the bond length.
Note: The possibility to make a mistake is that you may choose option C. But in 1,3−dichlorobenzene they are not parallel and hence they will not cancel out completely, it has some dipole moment.
