Question
Question: Which of the following compounds has three \(1^\circ\), one \(2^\circ\) and one \(3^\circ\) carbon? ...
Which of the following compounds has three 1∘, one 2∘ and one 3∘ carbon?
A.CH3(CH2)3CH3
B.CH3CH(CH3)CH3
C.CH3(CH2)2CH3
D.CH3CH(CH3)CH2CH3
Solution
The carbon can be divided into primary 1∘, secondary 2∘, tertiary 3∘and quaternary 4∘ depending upon the number of carbon atoms attached to the single carbon atom by a single bond.
Complete step by step answer:
-When one carbon atom is attached directly to the carbon atom, then the carbon is said as primary carbon.
-When two carbon atoms are attached directly to the carbon atom, then the carbon is said as secondary carbon.
-When three carbon atoms are attached directly to the carbon atom, then the carbon is said as tertiary carbon.
-When four carbon atoms are attached directly to the carbon atom, then the carbon is said as quaternary carbon.
CH3(CH2)3CH3
-The structure is shown below.
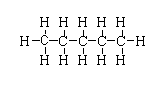
-In this compound, two primary carbon is present and three secondary carbon is present.
CH3CH(CH3)CH3
-The structure is shown below.
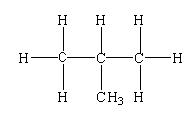
-In this compound, three primary carbon is present and one tertiary carbon is present.
CH3(CH2)2CH3
-The structure is shown below.
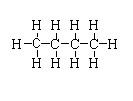
In this compound, two primary carbon is present and two secondary carbon is present.
CH3CH(CH3)CH2CH3
The structure is shown below.
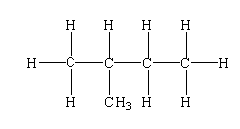
In this compound, one tertiary carbon, three primary carbon, and one secondary carbon are present.
Thus, the compound which has three 1∘, one 2∘ and one 3∘ carbon is CH3CH(CH3)CH2CH3.
Therefore, the correct option is D.
Note:
The carbon cannot exceed the quaternary carbon because to have five substituent carbon atoms, ten electrons are needed around the carbon atom which is not possible because in the valence orbital only eight electrons can be present according to the octet rule.
