Question
Question: Which of the following compounds has the lowest nitrogen – nitrogen bond length? \({{N}_{2}},{{N}_...
Which of the following compounds has the lowest nitrogen – nitrogen bond length?
N2,N2H2,N2F2 .
A. N2
B. N2H2
C. N2F2
D. All have equal bond length.
Solution
We know that the length of a single bond is high when compared to length of the double bond and triple bonds. The length of the double bond is high when compared to the length of the triple bond.
Complete step by step answer:
- In the question it is asked which molecule contains lowest nitrogen – nitrogen bond length in the given molecules.
- To know about the bond length we should know the structures of the given molecules.
- The structure of the nitrogen molecule (N2 ) is as follows.
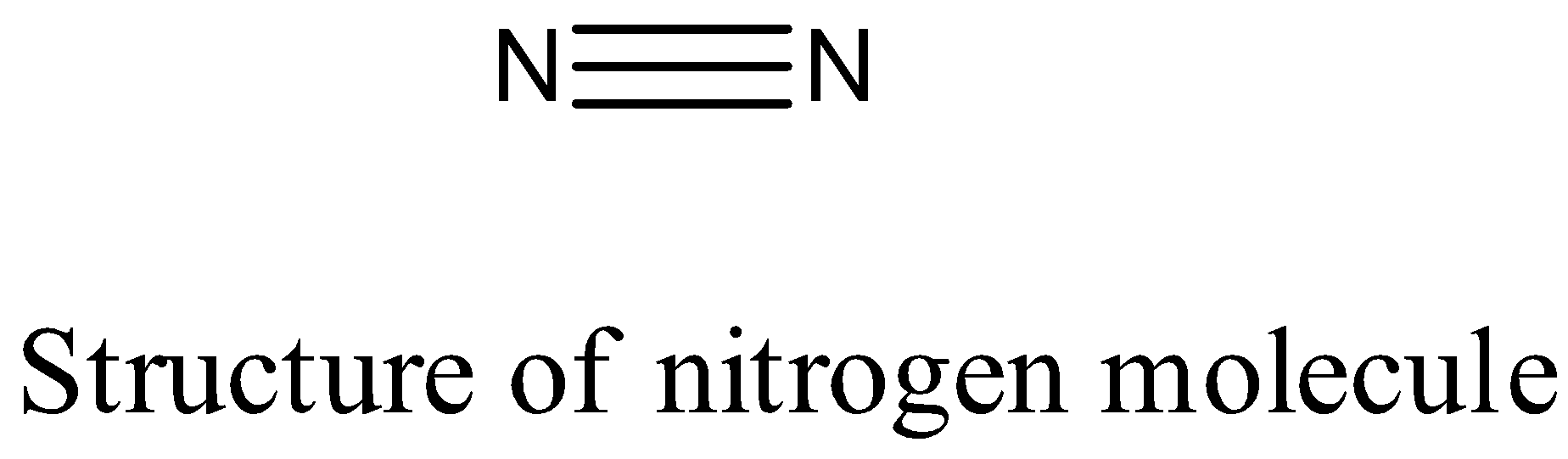
- The nitrogen – nitrogen bond length in nitrogen molecules is 109 pm.
-The structure of the hydrazine ( N2H2 ) is as follows.
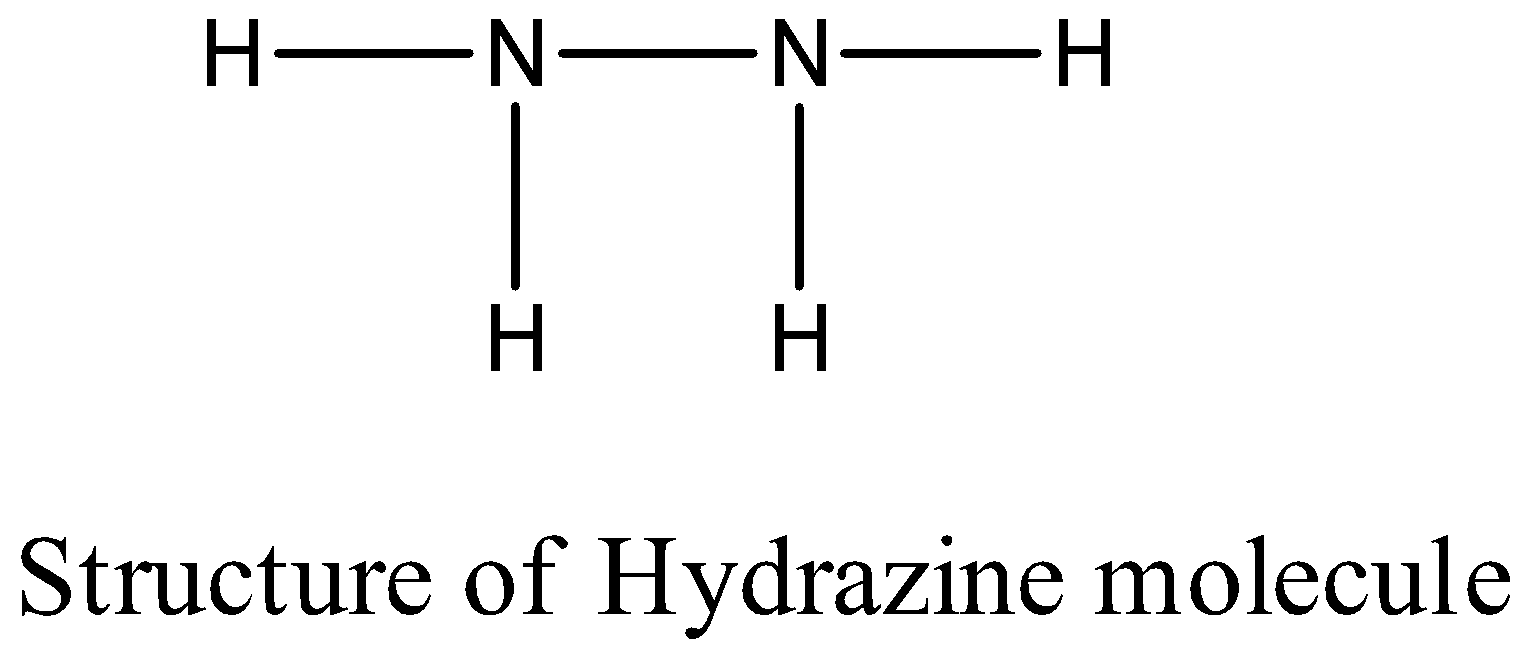
- The nitrogen – nitrogen bond length in the hydrazine molecule is 147 pm.
- Coming to the structure of N2F2 .
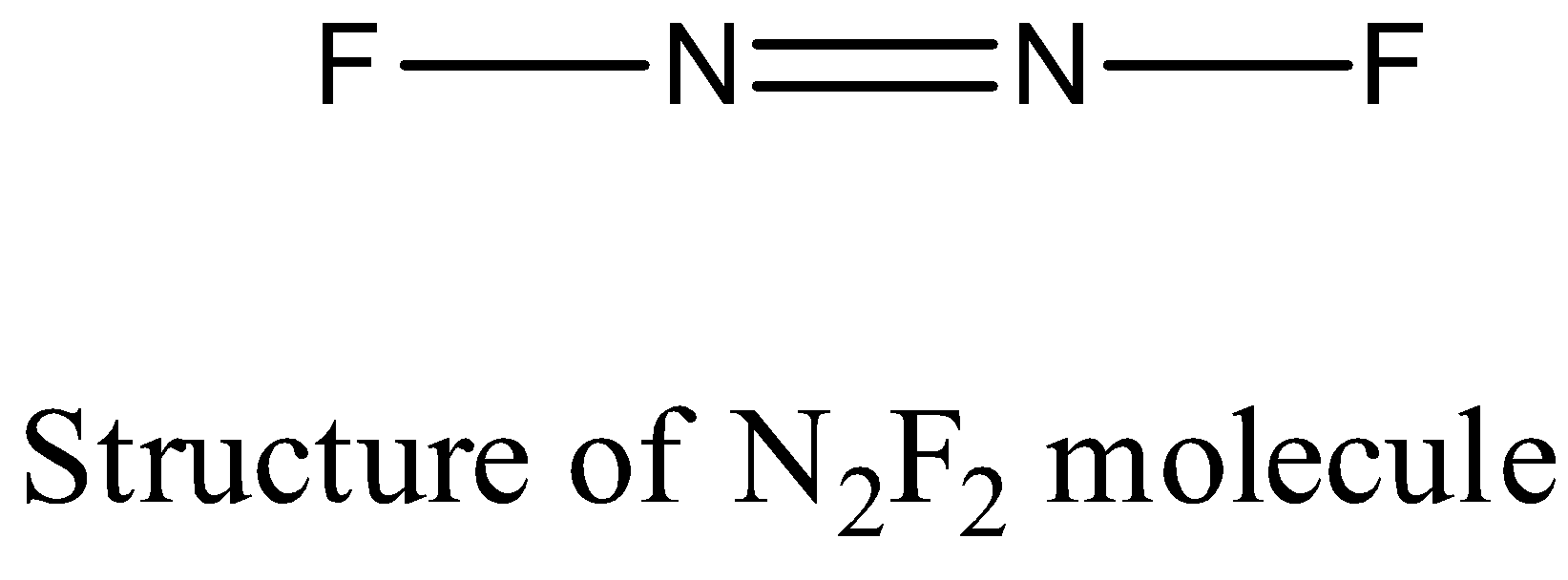
- The nitrogen – nitrogen bond length in ( N2F2 ) is 122 pm.
- Therefore the nitrogen – nitrogen bond lengths of the given molecules N2,N2H2, and N2F2 are 109 pm, 147 pm and 122 pm respectively.
- So, the nitrogen –nitrogen bond length is lowest in nitrogen molecules.
The correct option is option “A” .
Note: We know that the length of the triple bond is less and it is present in the structure of the nitrogen molecule. The strength of the triple bond is high when compared to the strength of the double and single nitrogen- nitrogen bond length.
