Question
Question: Which of the following compounds has linear shape? (A)\({{C}_{2}}{{H}_{4}}\) (B)\({{C}_{2}}{{H}...
Which of the following compounds has linear shape?
(A)C2H4
(B)C2H2
(C) CH4
(D) C3H6
Solution
Attempt this question finding the steric number of the carbon atom in each of the given compounds. As we know that for a compound to be linear, its hybridization must be sp which is possible when steric number is equal to 2.
Formula used:
Steric number = Number of atoms bonded to the central atom + number of lone pairs on the central atom.
Complete answer:
As we know that Steric Number of a molecule is used in Valence Shell Electron Pair Repulsion theory to determine the molecular geometry of that molecule i.e., it gives the electron pair arrangement for the geometry.
Below are the little hybridizations and their shape with respect to the steric number that will be required for this question:-
| Steric Number | Hybridization | Shape |
|---|---|---|
| 2 | sp | Linear |
| 3 | sp2 | Trigonal planar |
| 4 | sp3 | Tetrahedral |
-Now let us check the steric number of carbon in each and every molecule by making their structure and then further calculations:-
(A)C2H4
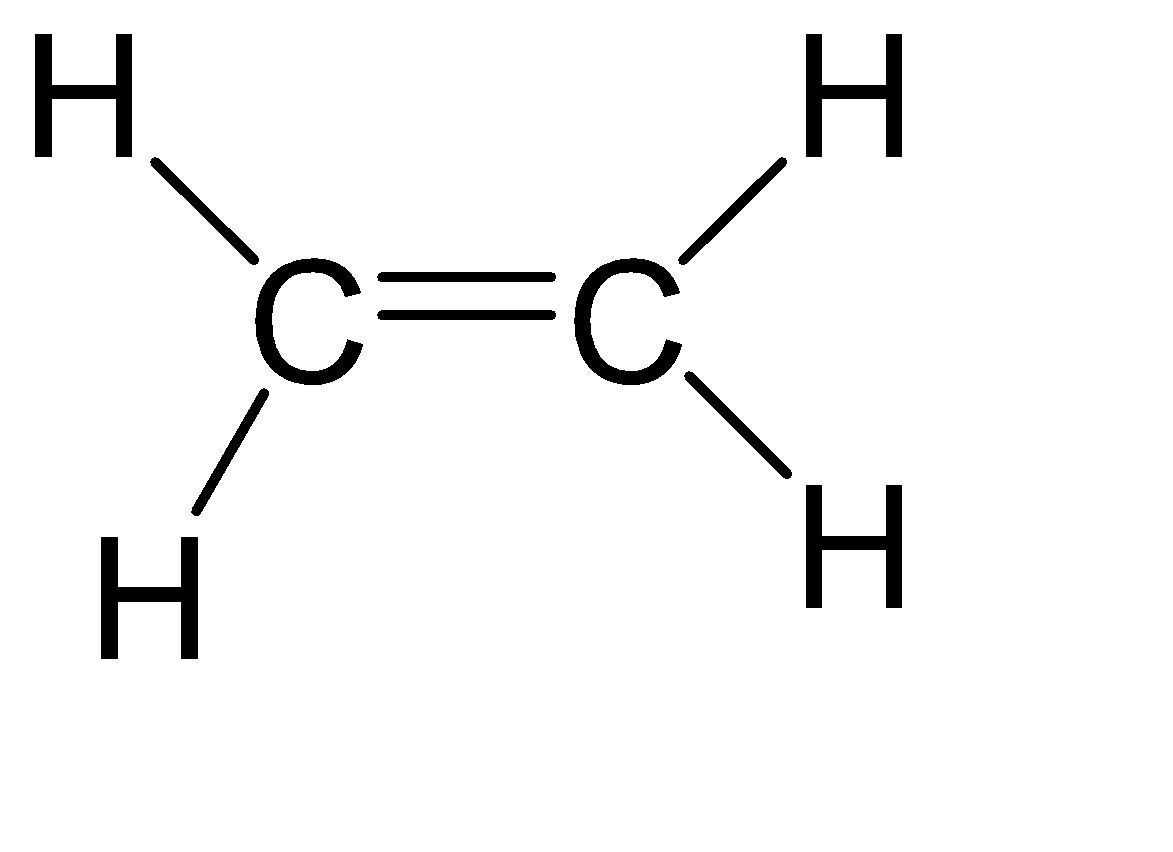
Since both carbons are the same, therefore we can calculate the steric number of any one of them.
Steric number = Number of atoms bonded to the central atom + number of lone pairs on the central atom.
Steric number = 3 + 0 = 3
From the above table, we can assume that its hybridization is sp2 and hence the shape is Trigonal Planar.
(B)C2H2
H−C≡C−H
Since both carbons are the same, therefore we can calculate the steric number of any one of them.
Steric number = Number of atoms bonded to the central atom + number of lone pairs on the central atom.
Steric number = 2 + 0 = 2
From the above table, we can assume that its hybridization is sp and hence the shape is Linear.
(C) CH4
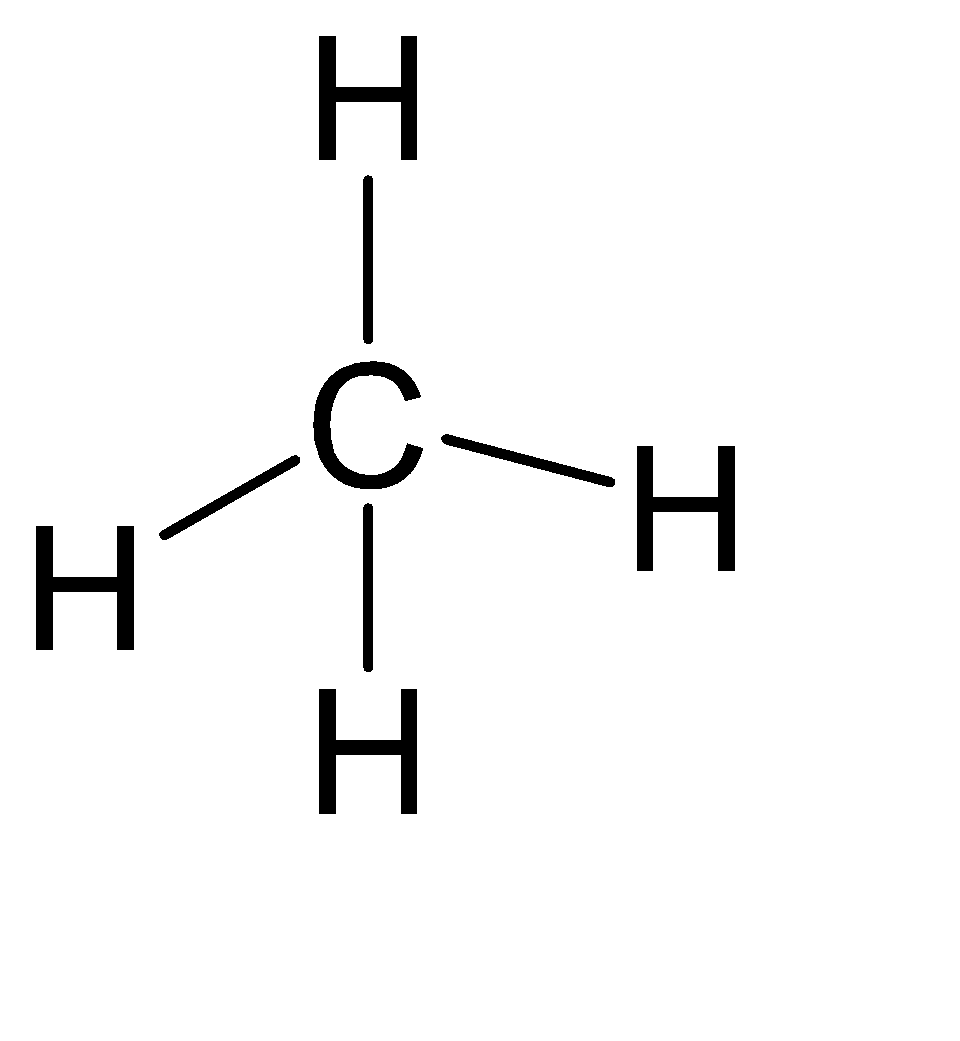
Steric number = Number of atoms bonded to the central atom + number of lone pairs on the central atom.
Steric number = 4 + 0 = 4
From the above table, we can assume that its hybridization is sp3 and hence the shape is Tetrahedral.
(D) C3H6
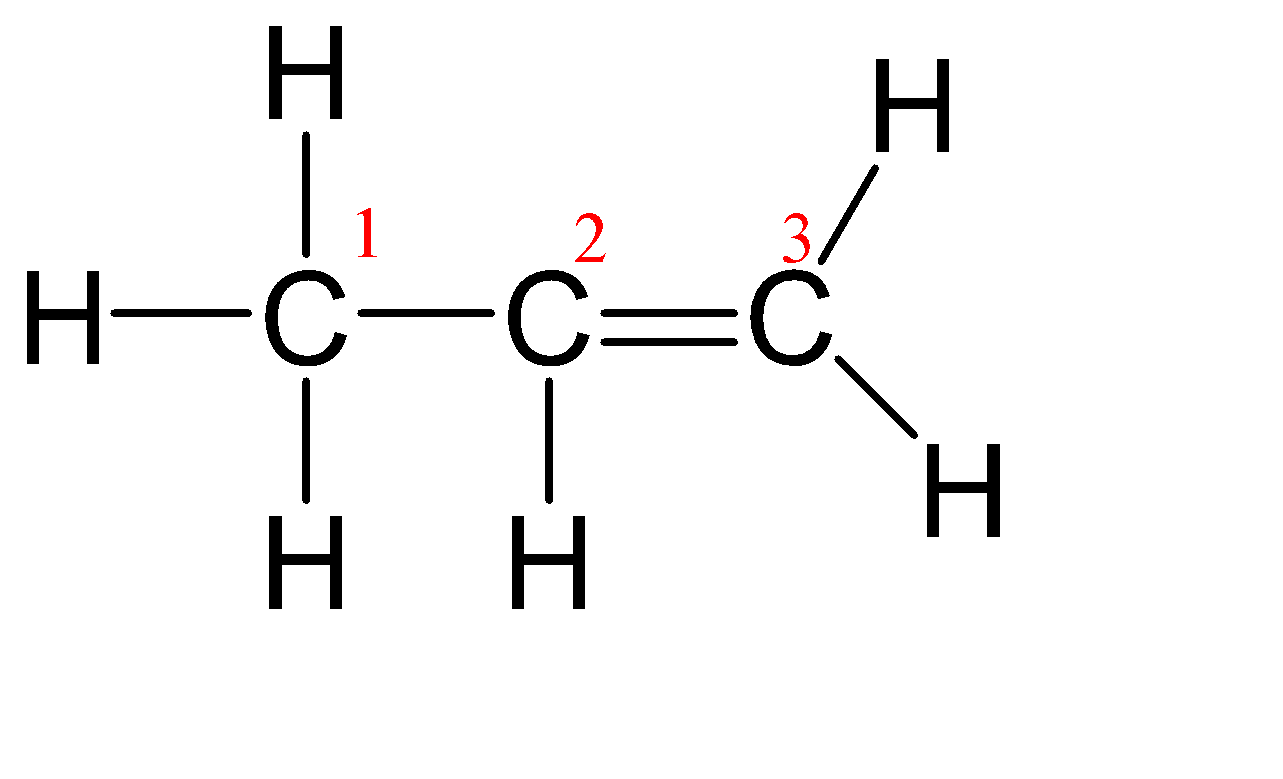
Since all the carbon have different environments so even if a single carbon has a shape other than linear then the whole molecule will not be considered as a linear molecule.
Steric number (C1 ) = Number of atoms bonded to the central atom + number of lone pairs on the central atom.
Steric number (C1 ) = 4 + 0 = 4
From the above table, we can assume that its hybridization is sp3 and hence the shape is Tetrahedral.
-Hence the correct answer is (B)C2H2
Note:
We can also calculate steric number by using the number of sigma bonds as follows:
Steric number = Number of sigma bonds around the central atom + number of lone pairs on the central atom.
-In case ofC3H6, there is possibility of another structure as well:-
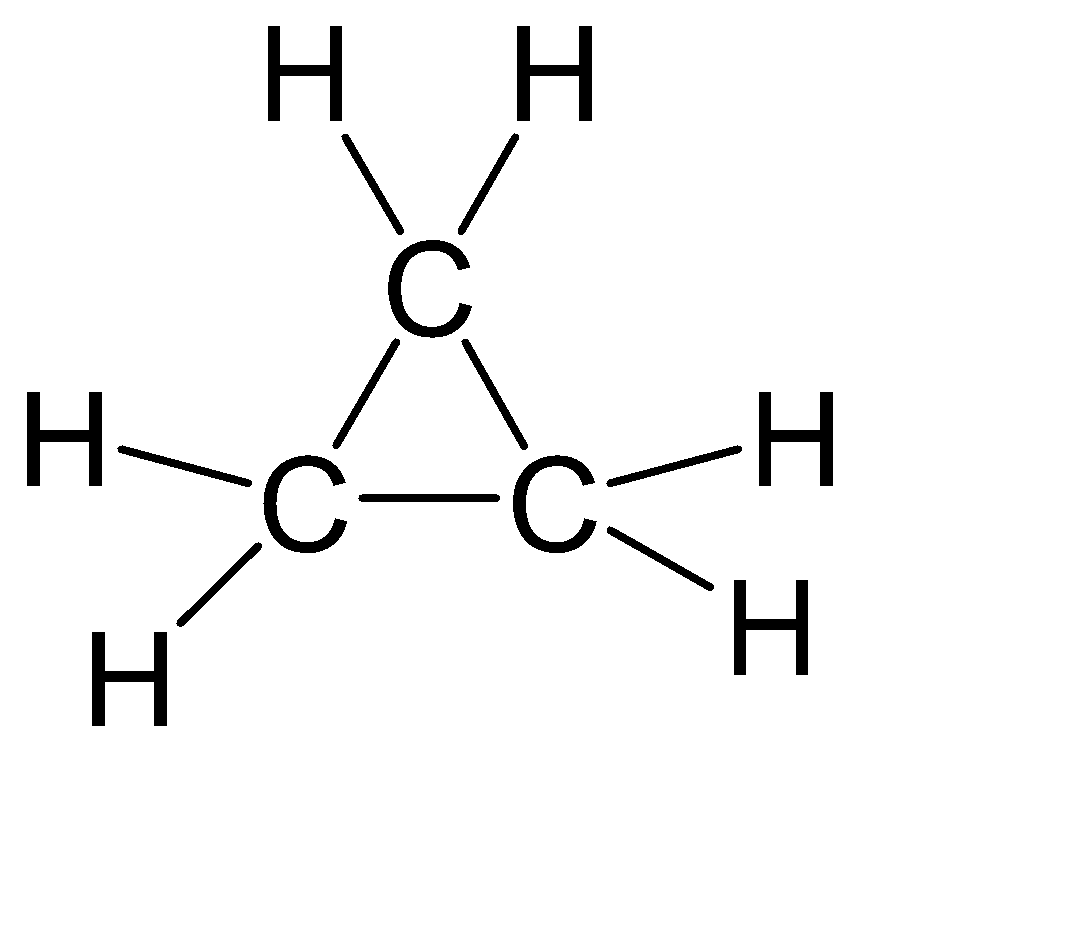
Even this structure is not linear hence the answer remains the same.
