Question
Question: Which of the following compounds has incorrect IUPAC nomenclature? This question has multiple correc...
Which of the following compounds has incorrect IUPAC nomenclature? This question has multiple correct options
A. CH3CH2COOC2H5← ethyl butanoate
B. CH3CH(CH3)CH2CHO ← ethyl butanoate
C. CH3CH(CH3)COCH2CH3← 2-methyl-3-pentanone
D. CH3CH(CH3)CH(OH)CH3← 2-methyl-3-butanol
Solution
IUPAC names are combined with the primary prefix, root word, and primary suffix. By this, each compound can be named unique. Primary prefixes are used to indicate the origin of the compound in IUPAC nomenclature. Prefixes are used to differentiate the cyclic and acyclic molecules in IUPAC nomenclature.
Complete step by step answer:
In the IUPAC nomenclature case, the root word is the number of total carbons in the longest chain of that compound.
| No. of carbons | Root word |
|---|---|
| 1 | meth |
| 2 | eth |
| 3 | prop |
| 4 | but |
| 5 | pent |
… and so on.
The primary suffix is used to differentiate between the saturated compounds (Alkanes) and unsaturated compounds (Alkene and Alkynes).
| compound | suffix |
|---|---|
| Alkane | ane |
| Alkene | Ene |
| Alkyne | Yne |
If there is more than one suffix. Then one of those suffixes is considered as the secondary suffix.
Example: Methanol (Alkanol), here ‘ol’ is a secondary suffix.
The primary prefixes are used to differentiate between cyclic compounds and noncyclic or chain compounds. For cyclic compound prefix s ‘cyclo’. if there are any side chains or groups are present then secondary prefixes like ‘methyl’, ‘ethyl’, ‘propyl’, ‘isopropyl’ are used.
Now according to this IUPAC nomenclature rules, in case of CH3CH2C00C2H5 the IUPAC name should be, ethyl propanoate. On the other hand, the IUPAC name of CH3CH(CH3)CH(OH)CH3 should be 3-methyl-2-butanol.
So, the given IUPAC names of these two compounds are wrong.
Therefore, the correct options are, A and D.
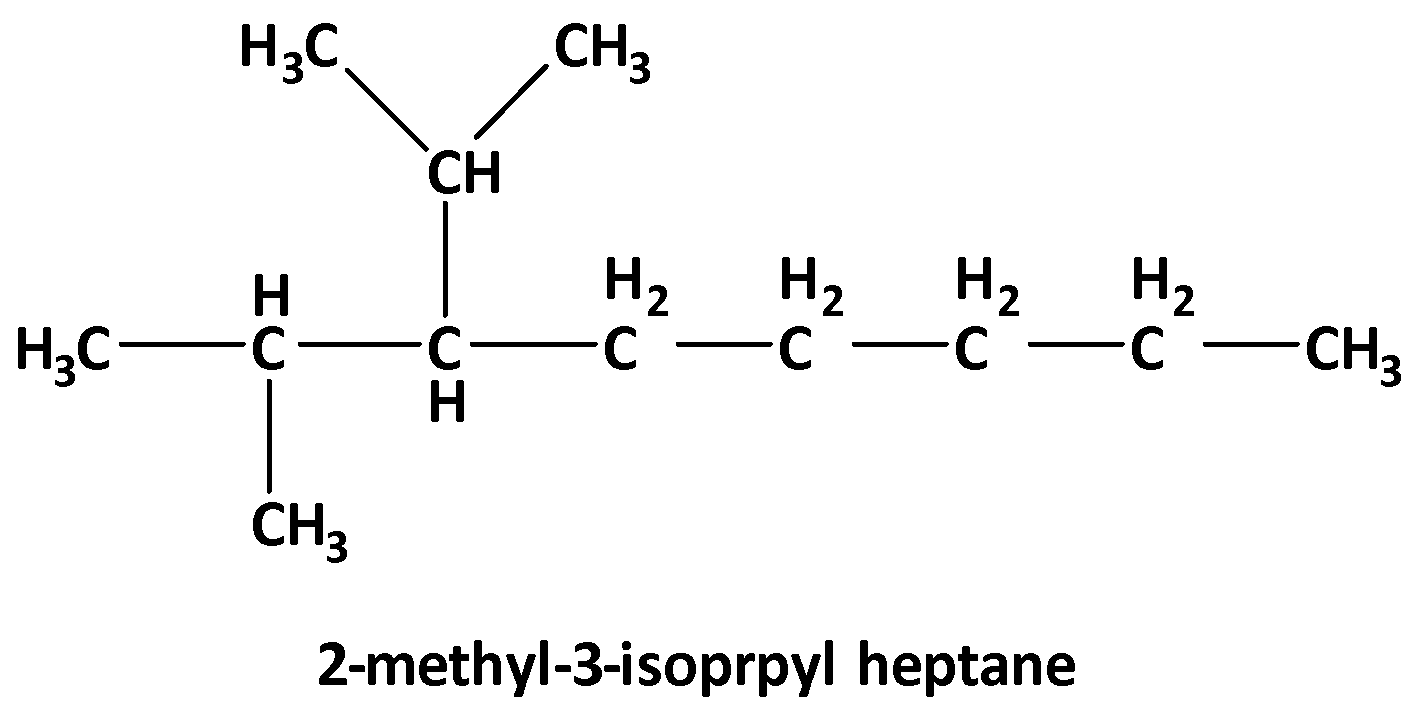
Note: Considering the IUPAC nomenclature, the structure of 2-methyl-3-isopropyl heptane is
“heptane” is for seven carbon in the longest chain, and it is from the group of alkanes that are all sigma bonds. “2-methyl” means there is a methyl group at 2 positions and “3-isopropyl” means there is an isopropyl group at 3 positions. So, the structure is as follows,