Question
Question: Which of the following compounds has a zero dipole moment? A. 1. 1 Dichloroethylene B. Cis 1.2...
Which of the following compounds has a zero dipole moment?
A. 1. 1 Dichloroethylene
B. Cis 1.2 dichloroethylene
C. Trans-1,2—dichloroethylene
D. Both 2 and 3
Solution
Dipole moment is a quantity that describes two charges that are separated by a distance. It is a quantity that we can measure the quantity in the lab for a molecule to determine the bond distance, size of the partial charges of the molecules. The dipole moment is also measuring the polarity of the molecule.
Complete step by step answer:
The structure of and Cis 1.2 dichloroethylene is shown below,
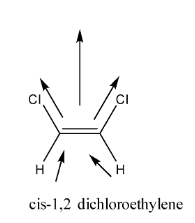
In the given structure it is clear that C is 1.2 dichloroethylene shows dipole moment. As chlorine is more electronegative than hydrogen, the overall dipole moment is towards chlorine.
On the other hand, structures of Trans-1,2—dichloroethylene and 1. 1 Dichloro ethylene is shown below.
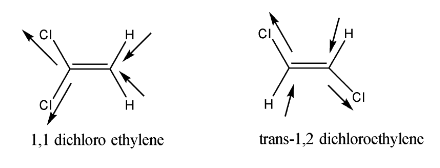
From the given structures it is clear that Trans-1,2—dichloroethylene and 1. 1 Dichloroethylene has no dipole moment, as the bond dipoles of each structure get nullified with each other.
Note: A molecule is called to be a nonpolar molecule if there is an equivalent sharing of electrons between two atoms of a molecule which is diatomic in nature, or if there is symmetry in the polar bonds of a significantly complex molecule. As well as when the dipole moment is zero of a molecule then it is called a nonpolar molecule. And if there is a dipole moment then it is called a polar molecule.
