Question
Question: Which of the following compounds has a P−P bond? (A) \({{\left( HP{{O}_{3}} \right)}_{3}}\) (B)...
Which of the following compounds has a P−P bond?
(A) (HPO3)3
(B) H4P2O6
(C) H4P2O7
(D) H4P2O5
Solution
In a P−P bond two phosphorous atoms are bonded with each other and form a bridge between the atoms. The bond between two phosphorus atoms is a covalent bond which is formed by the sharing of electrons by different atoms in a molecule.
Complete step by step solution:
- Among the given options H4P2O6 contains the P−P bond. The chemical name ofH4P2O6 is hypophosphoric acid. The acid has an oxidation state of +4. The structure of hypophosphoric acid is given below
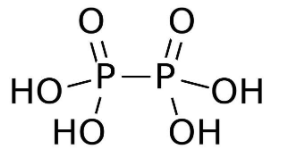
- As we can see in the structure of H4P2O6 a single P−P bond is present. Also it contains two P=O bonds and four P−OH bonds .In the structure of H4P2O6 the P−O bonds have a bond length of 151 pm and P−P bond has a bond length of 219 pm.
- H4P2O6 is a mineral acid and in the solid state it is present as dihydrate which has the molecular formulaH4P2O6.2H2O. It contains oxonium ions and is formulated as[H3O+]2[H2P2O6]2−.
From the above discussions it’s clear that the compound hypophosphoric acid H4P2O6 has a P−P bond in it.
Therefore, the answer is option (B). H4P2O6.
Additional information:
The hypophosphoric acid is generally used as reducing agents, wetting agents, as a bleaching agent and as a stimulant in pharmaceutical agents.
Note: Keep in mind that the hypophosphoric acid is a triprotic acid. Also do not confuse Hypophosphorous acid with Hypophosphoric acid. Hypophosphorous acid or phosphinic acid has the molecular formula H3PO2 whereas Hypophosphoric acid has the molecular formula H4P2O6. In addition to this, there are also other oxyacids of phosphorus such as Phosphorus acid, Diphosphoric acid (Pyrophosphoric acid), Peroxophosphoric acid etc.
