Question
Question: Which of the following compounds gives the Reimer-Tiemann reaction ? A. 
B. 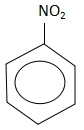
C. 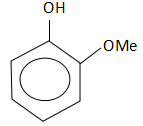
D. 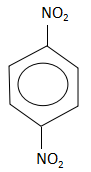
Solution
The Reimer–Tiemann reaction is a chemical reaction used for the ortho- formylation of phenols with the simplest example being the conversion of phenol to salicylaldehyde. There is an ortho attack of the carbene intermediate formed in the reaction as carbene acts as reactive and strong electrophile.
Complete step by step answer:
In the Riemer- Tiemann reaction, a phenol gets converted to salicylaldehyde in the presence of chloroform which yields a carbene intermediate that attacks the electron rich phenol at its electron dense site. The reaction involved here is:
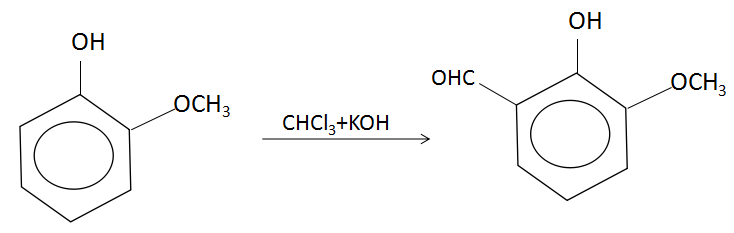
Chloroform is deprotonated (loss of proton) by a strong base (normally hydroxide) to form the chloroform carbanion which will quickly alpha-eliminate to give dichlorocarbene. This is the primary or principal reactive species. Along with this, the hydroxide ions will also deprotonate the phenol to give a negatively charged phenoxide ion. The negative charge is delocalized into the aromatic ring, making it far more nucleophilic. Nucleophilic attack on the dichlorocarbene gives an intermediate dichloromethyl substituted phenol. After basic hydrolysis, the desired product is formed. Because of its two electron-withdrawing chlorine groups, carbene is a highly electron deficient species and is attracted to the electron rich phenoxide. This interaction favors selective ortho-formylation.
So, the correct answer is Option C.
Note: The Reimer–Tiemann reaction can be altered to yield phenolic acids by substituting the chloroform (CHCl3 ) with carbon tetrachloride (CCl4 ). For instance, the altered reaction with phenol would yield salicylic acid rather than the expected product, salicylaldehyde.
