Question
Question: Which of the following compounds give(s) iodoform test ?...
Which of the following compounds give(s) iodoform test ?
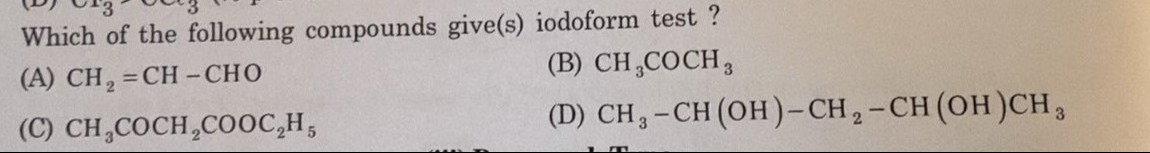
CH2=CH-CHO
CH3COCH3
CH3COCH2COOC2H5
CH3-CH(OH)-CH2-CH(OH)CH3
(B), (C), (D)
Solution
The iodoform test is given by compounds containing the methyl ketone group (CH3CO−) or a methyl secondary alcohol group (CH3CH(OH)−). Under the basic conditions of the test, the methyl secondary alcohol group is oxidized to the methyl ketone group.
Let's examine each compound:
(A) CH2=CH−CHO (Acrolein): This compound is an α,β-unsaturated aldehyde. It does not contain a CH3CO− group or a CH3CH(OH)− group. Therefore, it does not give the iodoform test.
(B) CH3COCH3 (Acetone): This compound contains a CH3CO− group. Therefore, it gives the iodoform test. CH3COCH3+3I2+4NaOH→CHI3↓+CH3COONa+3NaI+3H2O
(C) CH3COCH2COOC2H5 (Ethyl acetoacetate): This compound contains a CH3CO− group. Therefore, it gives the iodoform test. CH3COCH2COOC2H5+3I2+4NaOH→CHI3↓+CH3COONa+CH2(Na)COOC2H5+3NaI+3H2O
(D) CH3−CH(OH)−CH2−CH(OH)CH3 (Pentane-2,4-diol): This compound contains two CH3CH(OH)− groups. Under the reaction conditions, these are oxidized to CH3CO− groups, forming pentane-2,4-dione (CH3COCH2COCH3). Pentane-2,4-dione contains two CH3CO− groups and gives the iodoform test. CH3CH(OH)CH2CH(OH)CH3I2,OH−CH3COCH2COCH3 CH3COCH2COCH3+6I2+8NaOH→2CHI3↓+NaOCOCH2COONa+6NaI+6H2O
Therefore, compounds (B), (C), and (D) give the iodoform test.
