Question
Question: Which of the following compounds exhibit tautomerism: A.Nitromethane B.Nitroethane C.Nitrobenz...
Which of the following compounds exhibit tautomerism:
A.Nitromethane
B.Nitroethane
C.Nitrobenzene
D.A,b
Solution
We know that isomerism is the phenomenon in which two or more compounds have the same chemical formula but different structures. The compounds are known as isomers. There are different types of isomers, such as, position isomers, tautomers, chain isomers etc.
Complete step by step answer:
Let’s first understand functional isomerism. The isomers in functional isomerism differ with respect to the nature of the functional groups and hence belong to different families. For example,C2H6O represents two functional isomers namely ethanol and methoxymethane.
CH3−CH2−OH and CH3−O−CH3
Now, we discuss tautomerism in detail. Tautomers are actually functional isomers which exist simultaneously and also in dynamic equilibrium. The isomerism in this case is termed as tautomerism. It is of different types but the most common among them is the keto-enol tautomerism. This isomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,
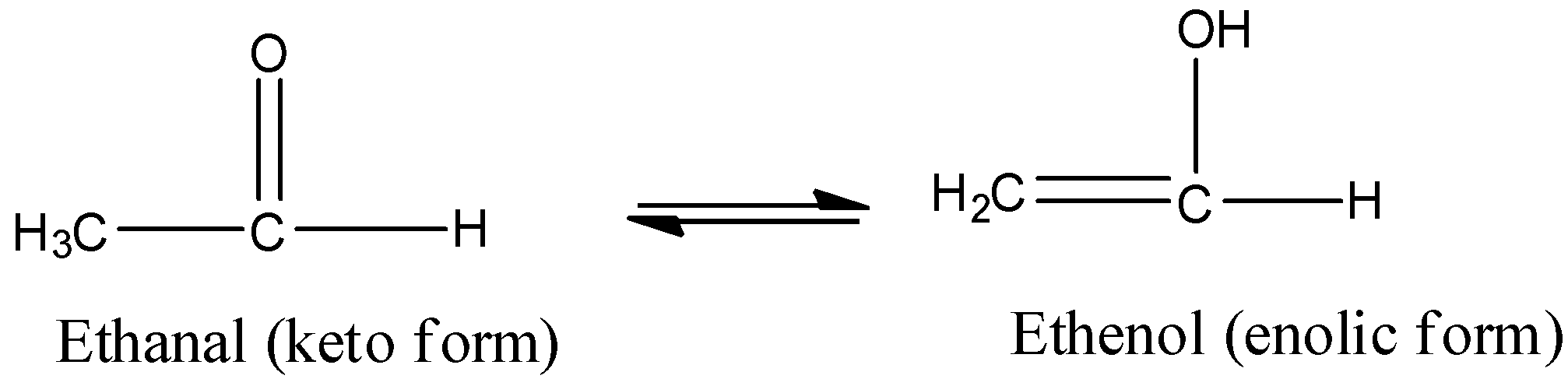
The condition for showing tautomerism by the compounds is the presence of alpha hydrogen in the compound.
Now, we have to check the presence of alpha hydrogen in the given compounds.
Option A is nitromethane. The structure of nitromethane is,
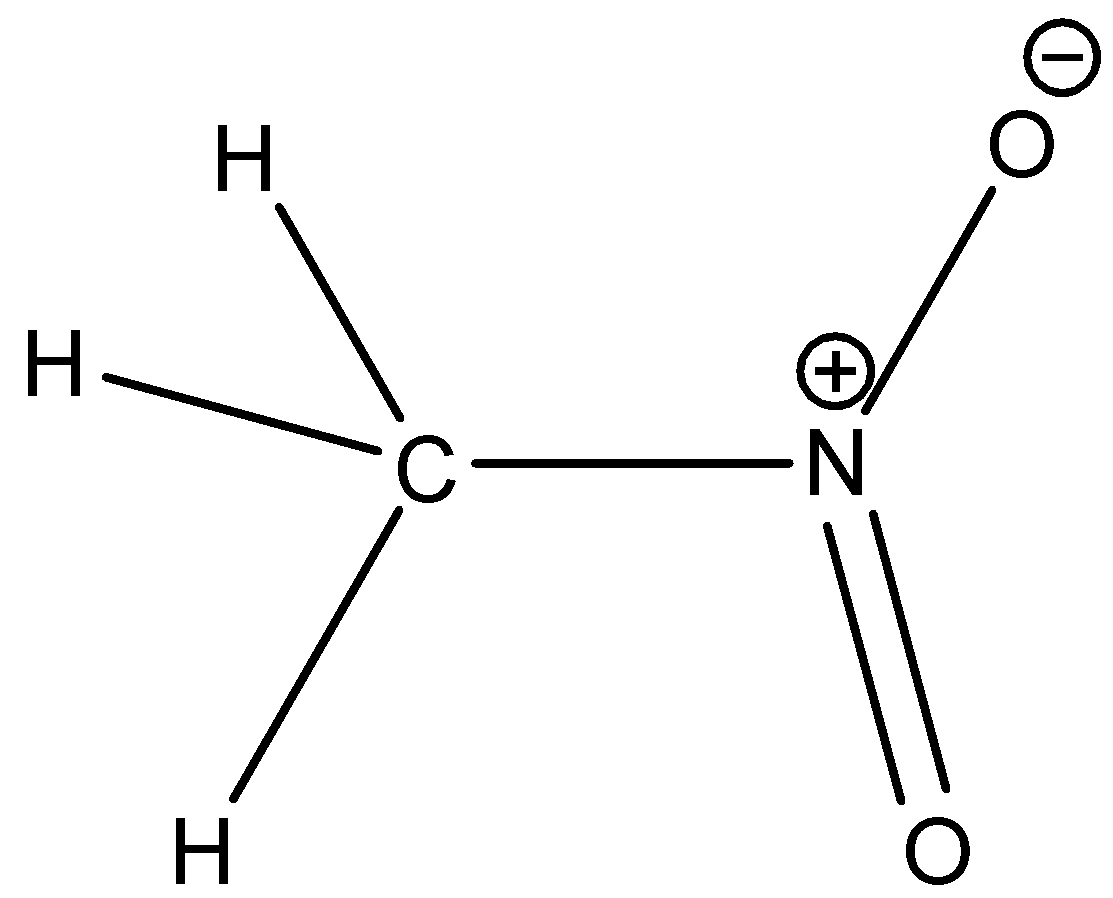
Here, alpha hydrogen is present. So, nitromethane shows tautomerism.
Option B is nitroethane.
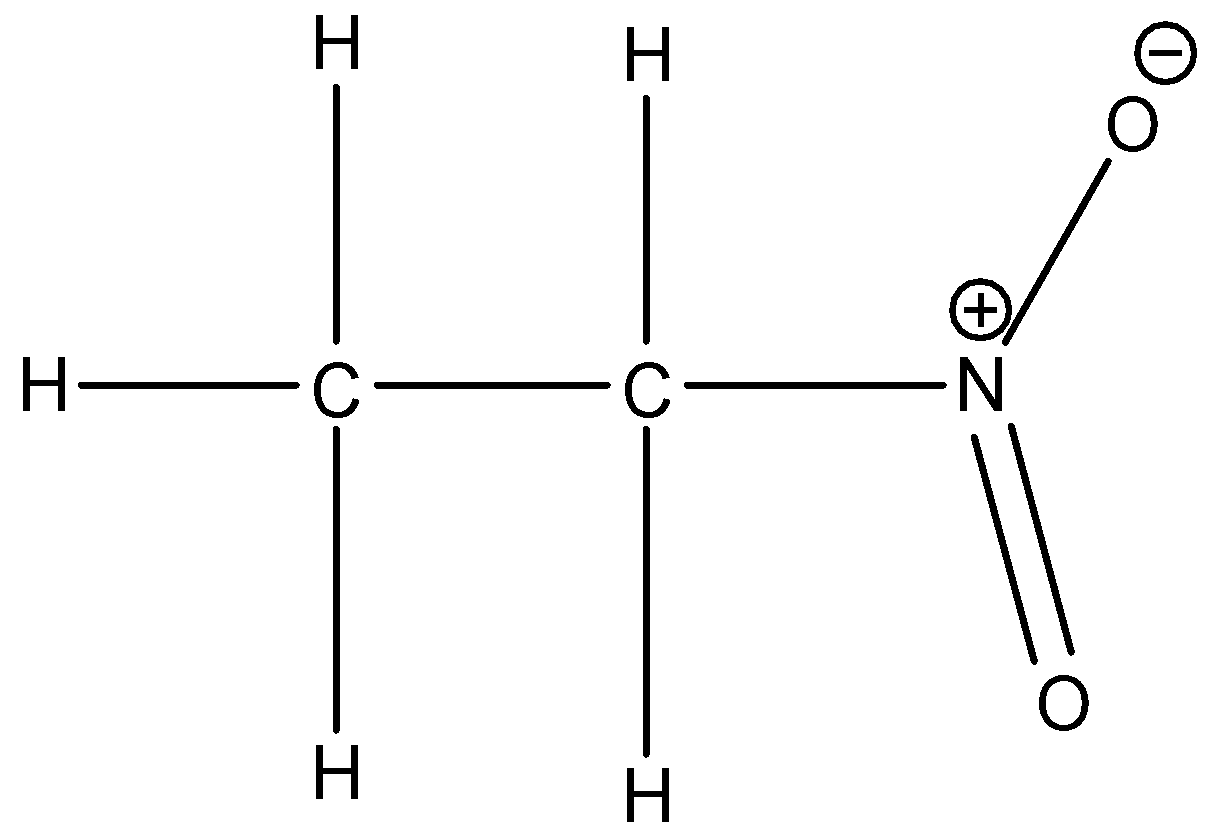
Here also, alpha hydrogen present. So, nitroethane also shows tautomerism.
Option C is nitrobenzene.
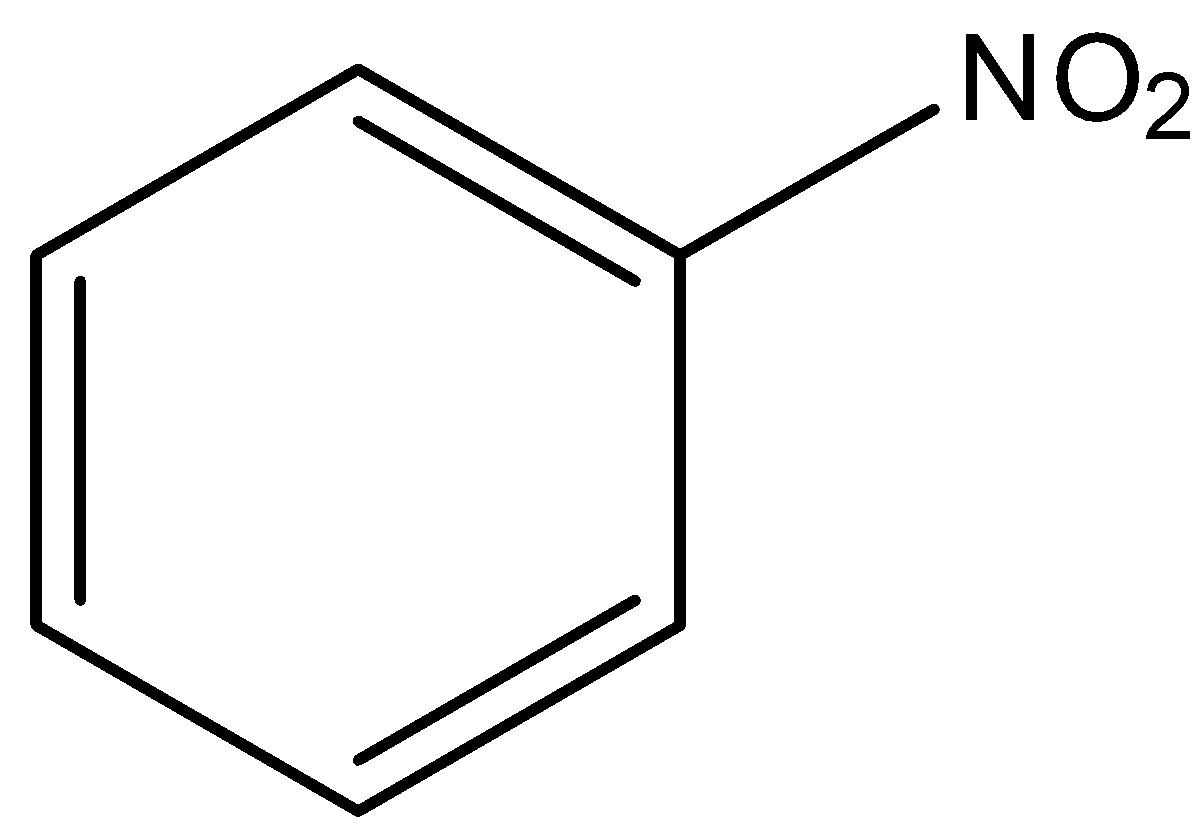
Here, no alpha hydrogen present. So, nitrobenzene does not show tautomerism.
Therefore, both nitromethane and nitroethane show tautomerism.
Hence the option D is the correct one.
Note: Always remember that an aldehyde or ketone shows tautomerism only when there is a presence of alpha hydrogen. The alpha hydrogen involved in 1,3 –migration. Tautomerism is not shown in absence of alpha hydrogen, such as benzaldehyde.
